- Volume 58 , Number 4
- Page: 666–73
Effects of lepromatous leprosy (LL) serum factor(s) on normal blood lymphocytes
ABSTRACT
To investigate the clastogenic activity of sera f rom leprosy patients, normal peripheral blood lymphocytes were cultured in both inactivated and noninactivated lepromatous leprosy (LL) sera. An increase in the frequency of chromosome aberrations was observed in normal lymphocyte cultures supplemented with both inactivated (5.2%) and noninactivated (5.0%) LL serum compared to that of cultures supplemented with normal human AB+ serum (2.4%). An enhanced frequency of sister chromatid exchanges (SCEs) was also observed in normal lymphocyte cultures supplemented with both inactivated (8.2 ± 3.85) (mean ± S.D.) and noninactivated (8.3 ± 4.61) LL serum compared to that of controls (6.8 ± 3.45). The normal blood lymphocyte cultures with LL serum have revealed a slow cell-cycle kinetics at a 48-hr incubation period, but a slightly faster proliferation rate was observed at 72 hr compared to cultures supplemented with normal human AB+ serum, indicating a depressive effect of LL serum on normal blood lymphocyte proliferation. The results obtained f rom the inactivated LL serum showed that the factors) which induce chromosomal damage, depress the mitotic index and the cell proliferation rate were not destroyed at 56șC. These results are the first documentation of cytogenetic effects of LL sera on normal human peripheral blood lymphocytes.RÉSUMÉ
Afin d'examiner l'activité clastogénique de serums de malades de la lèpre, des lymphocytes normaux de sang périphérique ont été mis en culture dans des serums de patients lépromateux (LL) inactivés et non inactivés. Une augmentation de la fréquence des aberrations chromosomiques fut observée dans les culturcs de lymphocytes normaux additionnées de sérum LL tant inactivé (5.2%) que non inactivé (5.0%), comparée à celle observée pour des cultures additionnées de sérum humain normal AB+ (2.4%). Une fréquence augmentée d'échanges de chromatides soeurs fut aussi observée dans des cultures de lymphocytes normaux additionnées de serums LL aussi bien inactivés (moyenne ± D.S.: 8.2 ± 3.85) que non inactivés (8.3 ± 4.61) comparées à celles de témoins (6.8 ± 3.45). Les cultures de lymphocytes normaux avec du sérum LL ont montré une cinétique lente du cycle cellulaire après une période d'incubation de 48 h, mais un taux de prolifération légèrement plus rapide fut observé après 72 h, comparé aux cultures additionnées de sérum humain normal AB + , indiquant un effet dépresseur du sérum LL sur la prolifération des lymphocytes de sang normal. Les résultats obtenus à partir du sérum LL inactivé ont montré que les facteurs qui induisent des dommages chromosomiques, dépriment l'index mitotique et le taux de prolifération cellulaire, n'étaient pas détruits à 56șC. Ces résultats sont la première démonstration des effets cytogénétiques des sérums LL sur des lymphocytes de sang humain périphérique normal.RESUMEN
Para investigar la actividad clastogcnica dc los sueros de los pacicntcs con lepra, se cultivaron linfocitos dc sangre periférica normal con sueros (inactivados y no inactivados) de pacientes con lepra lepromatosa (LL). Se observó un aumento en la frecuencia de aberraciones cromosómicas en los cultivos de linfocitos normales suplementados tanto con los sueros LL inactivados (5.2%) como con los no inactivados (5.0%). La frecuencia de tales aberraciones observada en los cultivos suplementados con suero humano normal AB + fuc del 2.4%. También se observó un incremento en la frecuencia de intercambios entre cromátidas hermanas (ICH) en los cultivos de linfocitos normales suplementados con los sueros LL inactivados (8.2% ± 3.85%) y con los no inactivados (8.3% ± 4.61%). La frecuencia observada en los cultivos adicionados de suero normal fue del 6.8% ± 3.45%. Comparados con los cultivos de linfocitos suplementados con suero humano normal AB + , los cultivos suplementados con suero LL mostraron una lenta cinética de ciclo celular en un periodo de incubación de 48 h, pero exhibieron una velocidad de proliferación ligeramente más rápida a las 72 h, indicando un efecto depresivo del suero LL sobre la proliferación de los linfocitos de sangre normal. Los resultados obtenidos con los sueros LL inactivados mostraron que el factor que induce el daño cromosómico y que deprime el índice mitótico y la velocidad dc proliferación celular, es termoestable a 56șC. Estos resultados son la primer documentación de los efectos citogenéticos de los sueros LL sobre los linfocitos humanos de sangre normalSerum is an essential component in in vitro cell culture. It has been shown that some unknown factors capable of inducing chromosome breakage in normal lymphocytes are present in the plasma/serum of chromosome instability and immuno-depressed syndromes (14,18,21,30,31), in chronic active hepatitis (12), and in patients with lupus erythematosus (15). It is well recognized that some unknown factor(s) present in the blood plasma of leprosy patients are capable of inhibiting the in-vitro growth of autologous lymphocytes after mitogen or antigen stimulation (2,23,28). The role of this factor(s) in lepromatous leprosy (LL) serum in the mitogenic response of normal lymphocytes to phytohemagglutinin (PHA) has been studied by several investigators (3, 4, 19, 20, 22, 24, 27) Mehra , et al. (20) suggested that plasma from some patients appears to contain inhibitory factor(s) which, however, may not be universally present in plasma of all leprosy patients. Recently, confirming previous observations of other laboratories, an inhibitor of lymphocyte activation was found by Kerr, et al. (19) in the serum or plasma of the majority of patients with lepromatous leprosy.
It is suggested that the inhibition by certain leprosy sera of the response of normal peripheral blood lymphocytes to stimulation with PHA was due to a decrease in the number of cells responding to mitogen, while the proliferation kinetics of cells which did respond were similar to those cultured in normal serum (19,27). Bullock and Fasal (5) and Nelson, et al. (24) have shown that some LL sera may be lymphocytotoxic. In contrast, Kerr, et al. (19) have shown that the putative inhibitory factor(s) present in LL sera are not cytotoxic. It is reported that the level and extent of inhibition by such factors seem to vary among different groups or types of leprosy patients. It has been suggested that these inhibitory responses may be due both to an inherent cellular defect and to the presence of factors in leprosy sera which inhibit lymphocyte activation (19). The inhibitory factor was found to be stable to heating at 56ºC for 30 minutes, but labile at 100ºC (19). This indicates that the factor may be short peptide molecules, since large peptides are denatured at 56ºC.
A wide range of substances has been suggested to be responsible for this inhibitory activity, although none has been characterized for their biochemical nature in detail. Holt, et al. (16) and Bjune (2) have suggested that a product of the leprosy bacillus is inhibitory, while Rook (29) reported that the inhibition is by a product derived from macrophages "overloaded" with mycobacteria. Recently, we have shown for the first time that there is a significant increase in the frequency of chromosome aberrations and sister chromatid exchanges (SCEs) in blood lymphocyte cultures of untreated leprosy patients (11).
In order to know whether any clastogenic activity is present in the sera of leprosy patients, the present study was designed to investigate the effect of sera from untreated LL patients on lymphocytes of normal individuals. The serum from only multibacillary (MB) patients, who are at a higher severity level of the disease and who show a higher rate of spontaneous (11) as well as induced chromosomal damage than paucibacillary (PB) patients (10) has been used.
MATERIALS AND METHODS
Patients and controls. Five untreated LL patients (age range 20-50 years; mean 41.4 years) and five age-matched healthy individuals (age range 23-45; mean 28.6 years) were selected for the study. The samples of peripheral venous blood from LL patients were allowed to clot in clean glass tubes, and the serum was separated out. One half of the serum was stored at - 70ºC, while the other half was first inactivated at 56ºC for 30 min and then stored as above.
Blood lymphocyte cultures. Heparinized whole blood (0.3 ml) was cultured according to the method of Arakaki and Sparkes (') in 5 ml of TC 199 medium (GIBCO Laboratories, Grand Island, New York, U.S.A.) supplemented with phytohemagglutinin-M (Difco Laboratories, Detroit, Michigan, U.S.A.), 20% serum, and antibiotics (penicillin and streptomycin) for 48 hr and 72 hr. 5-Bromodeoxyuridine (BrdU, 5 Mg/ml; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) was added to the initiation of the cultures. The cultures were set up as in the following two experimental conditions.
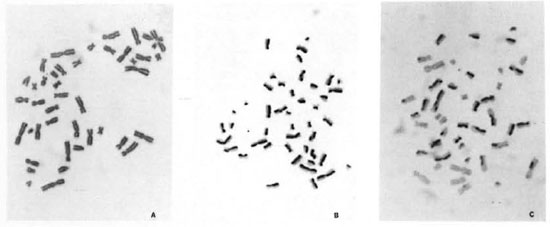
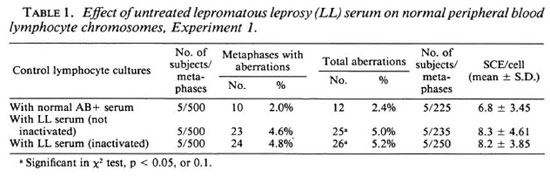
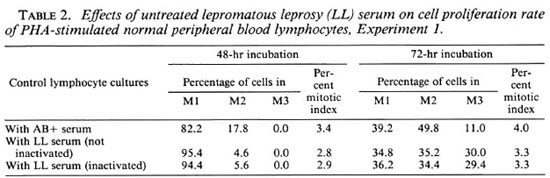
BrdU-labeling method. In view of the fact that mixing of different division metaphases may affect the correct estimate of chromosome aberrations (9), a BrdU-labeling method (26) has been used to ensure scoring of chromosome aberrations from only first-cycle metaphases (7,8) and SCEs from second-cycle metaphases. The addition of a low concentration of 5 µg/ml BrdU, a DNA base (thymidine) analog, for two consecutive replication cycles facilitates the differentiation between different division metaphases because of their characteristic differential staining patterns (Fig. 2A-C). After the first round of DNA replication, both chromatids of a chromosome are unifilarly substituted. This differential incorporation of BrdU produces light- and darkstained chromatids and any exchange (Fig. 1) between these can be detected unambiguously with clarity and high resolution under light-microscopy examination.
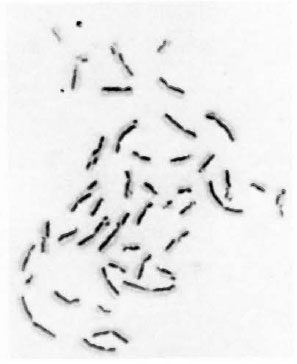
Fig 1. Metaphase showing 21 sister chromatoid exchanges.
Fig. 2. A-C. Mechanism of BrdU-labeling method. Metaphases showing cells in first (M1), second (M2),and third (M3+) division.
The cultures were grown in the dark at 37ºC. The flame-dried chromosome slides were treated with 50 µg/ml of Hoechst 33258 for 12 min at room temperature. After rinsing in distilled water, the slides were mounted with 2 x SSC (pH 6.8), and were exposed to bright sunlight for 2 to 3 hr. The slides were then rinsed in distilled water and stained in 2% Giemsa prepared in phosphate buffer (pH 6.8).
Experiment 1. Peripheral venous blood from five healthy individuals was cultured using LL serum in two sets: Set I with noninactivated serum; Set II with inactivated serum. As a control study, blood from these same five healthy individuals was cultured using inactivated normal human AB+ serum.
Experiment 2. Peripheral venous blood from donor "A," a healthy normal individual whose blood was previously cultured in AB+ serum and scored for spontaneous chromosome aberrations, SCEs and cell proliferation kinetics, was then cultured separately using sera from five LL patients in two batches. Batch 1 had non-inactivated LL serum and batch 2 had inactivated LL serum.
Scoring. One hundred first-cycle metaphases from 48-hr cultures were studied for scoring chromosome aberrations for each sample using ISCN criteria (33). Twenty to 50 second-cycle metaphases were scored from 72-hr cultures for SCEs in each subject, and the values were expressed as number of SCEs/cell. For analysis of the cellcycle kinetics, 100 metaphases at random were scored and the percentages of cells in the first (M1), second (M2), third and further cycles (M3+) were recorded for 48-hr and 72-hr incubation periods. The mitotic index was analyzed as cells in mitoses in a total of 1000 cells for each subject from 48hr and 72-hr incubation periods. The mitotic index was recorded as a percentage.
RESULTS
Experiment 1
The data on the frequencies of chromosome aberrations and SCEs obtained from normal lymphocyte cultures set up with noninactivated LL serum, inactivated LL serum and normal human AB+ serum are presented in Table 1. A significant (p < 0.05) increase in the frequency of chromosome aberrations was observed in normal lymphocyte cultures supplemented with inactivated LL serum (5.2%) and non-inactivated LL serum (5.0%), compared to that of cultures supplemented with normal human AB+ serum (2.4%). A significant enhanced frequency of SCEs was observed (Table 1, Fig. 1) in normal lymphocyte cultures supplemented with both inactivated (8.2 ± 3.85) (mean ± S.D.) and non-inactivated (8.3 ± 4.61) LL serum compared to that of control cultures set up with human AB+ serum (6.8 ± 3.45 SCEs/cell).
Cell proliferation kinetics. Table 2 summarizes the cell proliferation kinetics and mitotic indices for the 48-hr and 72-hr incubation periods. Use of the BrdU-Giemsa method facilitates easy identification of metaphases at the first (M1), second (M2), and third or further divisions (M3+) (Fig. 2A-C). There was a varied response in the cell proliferation rate. Three individuals' blood cultured in LL sera (inactivated and non-inactivated) showed a faster cell cycle at the 72-hr incubation period, while in two individuals, the cell proliferation rate was slow. The five control cultures showed a uniform cell proliferation rate.
The normal lymphocytes cultured with LL serum had a higher number of M1 cells at the 48-hr and a slightly increased number of M3 cells at the 72-hr incubation periods, indicating a slow cell-cycle kinetics at 48 hr of incubation but a somewhat faster cellcycle kinetics at 72 hr of incubation compared to cultures supplemented with human AB+ serum. Although not significant, the face values indicate a decrease in mitotic index at the 48-hr and 72-hr incubation periods in cultures with LL serum when compared to cultures with human AB+ serum, revealing a depressive effect of LL serum on normal lymphocyte proliferation.
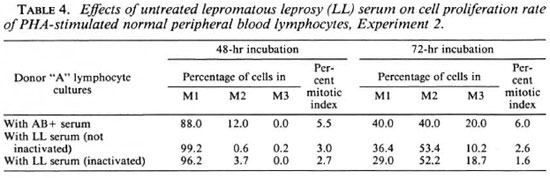
Experiment 2
Effects of LL sera on normal donor "A" lymphocyte cultures. The data on the effects of inactivated and nonactivated sera from five different LL patients on lymphocyte cultures of normal donor "A" are given in Table 3. There was a significant increase in the frequency of chromosome aberrations and SCEs in cultures supplemented with inactivated and non-inactivated LL sera compared to that of cultures with AB+ serum. But the frequency of chromosome aberrations and SCEs in cultures with inactivated and non-inactivated LL serum showed no significant difference. This indicates that inactivation of the LL sera does not destroy the factor(s) responsible for inducing chromosomal damage.
Cell proliferation kinetics. Table 4 gives the cell proliferation rate and mitotic indices of normal donor "A" lymphocyte cultures supplemented with both inactivated and non-inactivated sera from five different LL patients. The results obtained from inactivation of the LL sera are consistent with non-inactivated sera and show no significant change in the mitotic index or the cell proliferation rate. This, again, suggests that the depressive effect of the LL sera is not destroyed on inactivation at 56ºC.
DISCUSSION
It is well documented that normal lymphocytes do not respond well to mitogen stimulation when cultured in LL serum, and this is assessed by DNA synthesis rates using 3H-thymidine labeling (5, 24, 25). In the present study, utilizing the BrdU-labeling method, the effects of LL serum on normal lymphocytes in terms of the cell-cycle progression rate, chromosome aberrations, and SCEs have been evaluated. The observed cell proliferation rate, although initially slow at 48 hr, was comparable with controls at 72 hr, and was faster in a few individuals. It is not known why normal lymphocytes cultured in some LL sera show a faster rate of proliferation. It could be possible that the sera of these LL patients might contain insufficient or no inhibitory factor(s) (20). There was certainly a depression in the mitotic index in normal lymphocyte cultures supplemented with LL serum. These results are in accord with earlier findings by Potts, et al. (27) that the principal action of sera was to reduce the number of cells responding to mitogen, suggesting that LL sera contain some inhibitory factor(s). They also reported that these inhibitory factors are relatively nontoxic because the growth rate of the responding cells was unimpaired.
There was a significant increase in the frequency of chromosome aberrations; a slight, but consistent, increase in the frequency of SCEs was observed in normal lymphocytes cultured in LL serum, indicating that LL sera may contain some factor(s) which are clastogenic. These results are in accord with the cytogenetic studies on ataxia telangiectasia patients, which have shown that the plasma of these patients contains factors that can damage the chromosomes of normal cells (14, 21, 31).
In our experiment, inactivation of the various LL sera showed no significant change in the frequency of chromosome aberrations, SCEs, or cell proliferation rates, confirming the earlier findings of Kerr, et al. (19) that this inhibitory or clastogenic factor is stable to heating at 56ºC. It further indicates that the suspected factor may be short peptides which are not destroyed at this temperature. Since LL sera are particularly potent in inhibiting the action of PHA, it is possible that, by depressing T-cell activity in vivo, the serum factors may contribute to the immunologically nonspecific defects in cell-mediated immunity that are characteristic defects of LL patients. However, the significance of the serum inhibitor to lymphocyte activation in the immunopathology of leprosy is unknown (17). Holt, et al. (16) and Bjune (2) have demonstrated that the product of the leprosy bacillus is inhibitory. Whatever the factor which suppresses normal lymphocyte stimulation, it has not been shown, so far, to be genotoxic. However, in the absence of effects of sera of different blood groups on the induction of chromosome aberrations and SCEs (6), the observation of a significant increase in the frequency of aberrant metaphases and also of an enhanced level of SCEs in normal lymphocyte cultures supplemented with LL sera (instead of normal human AB+ sera) indicates that the factor(s) might be clastogenic or genotoxic.
It is quite likely that M. leprae may release into the circulation some substances which are genotoxic besides being mitotic and immunodepressive. It is also known that certain bacterial enzymes can act as DNases (32), which are thermostable and can induce lesions in the DNA. Alternatively, certain immune complexes or components of M. leprae could trigger an oxidative burst of the neutrophils which are present in the whole-blood samples used forculturing. This may cause production of hydrogen peroxide, superoxide, and lipid peroxides which could exert clastogenic activity. This may also lead to faster cell proliferation at the early stage (13). Whatever may be the possibility, there is certainly a clastogenic activity in the sera of LL patients when compared with that of controls. These results are the first documentation of cytogenetic effects of LL sera on normal lymphocytes. Further studies involving biochemical characterization of LL serum factors would better our understanding of the nature and functions of these factors.
Acknowledgments. We thank the JOURNAL'S referees for their invaluable comments and suggestions for improving the manuscript. We are grateful to the staff of the Department of Dermatology, St. John's Medical College Hospital, and the staff of Sumanahalli Rehabilitation Centre, Bangalore, for providing the blood samples of the patients. We gratefully acknowledge the advice and critical review of the work by Dr. (Sr.) Adelcia, A.C.; Dr. S. C. Rajendran and Dr. Elizabeth Jayaseelan of the same hospital, and the statistical advice of Mr. N. S. Murthy of the Institute of Cytology and Preventive Oncology (ICMR), New Delhi. This work was financed by Rameshwardas Birla Smarak Kosh, Bombay, India.
REFERENCES
1. ARAKAKI, D. T. and SPARKES, R. S. Microtechnique for culturing leukocytes from whole blood. Cytogenet. Cell Genet. 2(1963)57-60.
2. B JUNE , G. In vitro lymphocyte stimulation in leprosy simultaneous stimulation with Mycobacterium leprae antigens and phytohaemagglutinin. Clin. Exp. Immunol. 36(1979)479-487.
3. BJUNE, G., BARNETSON, R. ST. C, RIDLEY, D. S. and KRONVALL , G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of lesions. Clin. Exp. Immunol. 25(1976)85-94.
4. BULLOCK , W. E. Impairment of PHA and antigen induced DNA synthesis in leucocytes cultures from patients with leprosy. Clin. Res. 16(1968)328.
5. BULLOCK W. E., JR. and F ASAL, P. Studies of immune mechanisms in leprosy. III. The role of cellular and humoral factors in impairment of the in vitro immune response. J. Immunol. 106(1971)888-899.
6. DAS, B. C. Factors that influence formation of sister chromatid exchanges in human blood lymphocytes. CRC Crit. Rev. Toxicol. 19(1988)43-86(387 refs.).
7. DAS, B. C. and SHARMA , T. Enhanced frequency of chromosome aberrations in lymphocytes of male compared with female mutjacs after X-ray irradiation in vitro. Nature 290(1981)604-607.
8. DAS, B. C. and SHARMA , T. Blood lymphocyte culture system: quantitative analysis of X-ray induced chromosome aberrations in man, muntjac and cattle. Mutat. Res. 110(1983)111-139.
9. DAS, B. C. and SHARMA, T. The fate of X-ray induced chromosome aberrations in blood lymphocyte culture. Mutat. Res. 176(1987)93-104.
10. D'SOUZA, D., DAS, B. C. and THOMAS, I. M. Differential sensitivity of peripheral blood lymphocytes of untreated leprosy patients to mitomycin C. Mutat. Res. 240(1990)101-107.
11. D'SOUZA, D. and T HOMAS , I. M. Chromosome aberrations and sister chromatid exchanges (SCEs) in peripheral blood lymphocyte cultures of untreated leprosy patients. Lepr. Rev. 59(1988)121-125.
12. EL-ALFI, O. S., SMITH, P. N. and BIESELE , J. J. Chromosomal breaks in human leucocyte cultures induced by an agent in plasma of infectious hepatitis patients. Hereditas 52(1965)285-294.
13. E MERIT , I. Properties and action mechanism of clastogenic factors. In: Lymphokines. Pick, E., ed. London: Academic Press, 1983, vol. 8, pp. 413-424.
14. EMERIT, I., LEVY, A. and H OUSSET, E. Breakage factor in systemic sclerosis and protector effect of L-cysteinc. Hum. Genet. 25 (1974) 221-226.
15. EMERIT, I., MICHELSON, A. M., LEVY, A., CAMUS, J. P. and EMERIT , J. Chromosome breaking agent of low molecular weight in human systemic lupus erythematosus; protector effect of superoxide dismutase. Hum. Genet. 55(1980)341-344.
16. HOLT, P. G., FIMMEL, P. J., ROBERTS, L. M. and KEAST , D. Modification by soluble antigen of the immune response to mycobacterial infection. Infect. Immun. 16(1977)904-909.
17. HUSSEIN, Y. M., KERR, M. A. and BECK, J. S. The mechanism of action of the factor in leprosy scrum that inhibits the growth of mitogen stimulated normal human lymphocytes. Immunology 61(1987)125-129.
18. JASPERS, N. G. J. and BOOTSMA, D. Genetic heterogeneity in ataxia-telangiectasia studied by cell fusion. Proc. Natl. Acad. Sci. U.S.A. 79 (1982) 2641-2644.
19. KERR, M. A., H USSEIN, Y. N., POTTS, R. C, BECK, J. S. and S HERIFF , M. M. Characterisation of a factor in leprosy serum that inhibits the growth of mitogen stimulated normal human lymphocytes. Immunology 61(1987)117-123.
20. MEHRA, V. L., TALWAR, G. P., BALAKRISHNAN , K. and BHUTANI, L. K. Influence of chemotherapy and serum factors on the mitogenic response of peripheral leucocytes of leprosy patients to phytohaemagglutinin. Clin. Exp. Immunol. 12(1972)205-213.
21. MURNANE , J. P. and PAINTER, R. B. Complementation of the defects in DNA synthesis an irradiated and unirradiated ataxia-telangiectasia cells. Proc. Natl. Acad. Sci. U.S.A. 79(1982)1960-1963.
22. NATH, I., CURTIS , J., SHARMA, A. K. and TALWAR, G. P. Circulating T cell numbers and their mitogenic potential in leprosy-correlation with mycobacterial load. Clin. Exp. Immunol. 29(1977)393-400.
23. NAVALKAR , R. G. Immunology of leprosy. CRC Crit. Rev. Microbiol. 8(1980)25-47 (145 refs.).
24. NELSON, D. S. D., NELSON , M., T HURSTON, J. M., W ATERS, M. F. R. and P EARSON, J. M. H. Phytohacmagglutinin-induccd lymphocyte transformation in leprosy. Clin. Exp. Immunol. 9(1971)33-43.
25. NELSON, D. S., PENROSE, J. M., WATERS, M. F. R., PEARSON, J. M. H. and NELSON, M. Depressive effect of serum from patients with leprosy on mixed lymphocyte reactions. Influence of anti-leprosy treatment. Clin. Exp. Immunol. 22(1975)385-392.
26. PERRY, P. and WOLFF, S. New Giemsa method for the differential staining of sister chromatids. Nature 251(1974)156-158.
27. POTTS , R. C, SHERIFF , M. M., ROBERTSON, A. J., GIBBS, J. H., BROWN , R. A. and BECK, J. S. Serum inhibitory' factor in lepromatous leprosy: its effect on the pre-S phase cell cycle kinetics of mitogen stimulated normal human lymphocytes. Scand. J. Immunol. 14(1981)269-280.
28. REA, T. H. and LEVIN , N. E. Current concepts in the immunology of leprosy. Arch. Dermatol. 113(1977)345-352.
29. ROOK, G. A. W. The potentiating mitogenic and inhibitory effects on lymphocytes in vitro, in macrophages in the lymph nodes of mice "overloaded" with mycobacterial products. Clin. Exp. Immunol. 21(1975)163-172.
30. SHAHAM, M., BECKER, Y., and COHEN, M. M. A diffusable clastogenic factor in ataxia-telangiectasia. Cytogenet. Cell. Genet. 27(1980)155-161.
31. SHAHAM, M. and BECKER, Y. The ataxia telangiectasia clastogenic factor in a low molecular weight peptide. Hum. Genet. 58(1981)422-424.
32. SLACK, J. M. and SNYDER, I. S. Bacteria and Human Disease. Chicago, London: Year Book Medical Publishers, Inc., 1978, pp. 1-22.
33. STANDING COMMITTEE ON HUMAN CYTOGENETIC NOMENCLATURE. An International System for Human Cytogenetic Nomenclature. Harnden, D. G. and Klingcr, H. P., eds. Basel, New York: Karger, 1985, vol. 6.
1. Ph.D., Professor of Anatomy, Division of Human Genetics, Department of Anatomy, St. John's Medical College, Bangalore 560034, India.
2. M.S., Professor of Anatomy, Division of Human Genetics, Department of Anatomy, St. John's Medical College, Bangalore 560034, India.
3. Ph.D., Chief, Division of Molecular Oncology, Institute of Cytology and Preventive Oncology (ICMR), Maulana Azad Medical College Campus, New Delhi 110002, India.
Reprint requests to: Dr. (Sr.) Doris D'Souza, A.C., Patna Women's College, Bailey Road, Patna 800001, Bihar, India.
Received for publication on 7 September 1989.
Accepted for publication in revised form on 3 April 1990.