- Volume 58 , Number 4
- Page: 674–80
Effect of presensitization with BCG and Mycobacterium leprae on granuloma formation to M. leprae
ABSTRACT
Granulomas which develop in draining lymph nodes, following the intradermal injection of cobalt-irradiated Mycobacterium leprae into the ear of the guinea pig 2 and 5 weeks earlier, were studied in animals which had been presensitized with BCG vaccine or M. leprae and compared with granulomas that developed in previously unsensitized guinea pigs. Presensitization with mycobacteria accelerated the development of the granulomas. Granulomas in previously unsensitized guinea pigs were found ultrastructurally to contain phagocytosing macrophages similar to those in lepromatous leprosy, and M. leprae presensitization did not alter the type of granuloma found. Those in BCG-presensitized guinea pigs contained secretory epithelioid cells with rough endoplasmic reticulum similar to those found in borderline tuberculoid leprosy or reversal reactions. The significance of these findings in relation to the current use of vaccines in leprosy is discussed.RÉSUMÉ
Les granulomes qui se développent au niveau des ganglions lymphatiques, à la suite de l'injections intradermique 12 et 5 semaines auparavant, de Mycobacterium leprae irradié au Cobalt dans l'oreille de cobayes, ont été étudiés chez des animaux qui avaient été sensibilisés avec le vaccin BCG ou M. leprae, et comparés aux granulomes développés par des cobayes qui n'avaient pas été sensibilisés antérieurement. La sensibilisation antérieure avec des mycobacteries a accéléré la développement des granulomes. On a montré que les granulomes des cobayes non sensibilisés antérieurement contenaient des macrophages phagocytcurs semblables à ceux existant dans la lèpre lépromateuse, et la sensibilisation antérieure par M. leprae ne modifia pas le type de granulomes découverts. Les granulomes des cobayes pré-sensibilisés du BCG contenaient des cellules secrétrices épithélioïdes avec un reticulum cndoplasmique rugueux, semblables à celles trouvées dans la lèpre borderline tuberculoïde ou dans les réactions d'inversion. La signification des ces découvertes en relation avec l'utilisation actuelle de vaccins pour la lèpre est discutée.RESUMEN
Se inoculó intradermicamente Mycobacterium leprae irradiado con cobalto en la oreja de cobayos. Dos y cinco semanas después se estudiaron los granulomas desarrollados en los ganglios linfáticos regionales de animales que habían sido presensibilizados con BCG o con M. leprae y se compararon con los granulomas que se desarrollaron en cobayos sin presensibilizar. La presensibilización con micobacterias aceleró el desarrollo de granulomas. Los granulomas en cobayos no presensibilizados contuvieron macrófagos fagocitantes ultraestructuralmente similares a aquellos de la lepra lepromatosa; la presensibilización con M. leprae no alteró el tipo de granuloma encontrado. Los granulomas en los cobayos presensibilizados con BCG contuvieron células epitelioides con retículo endoplásmico rugoso similares a aquellas encontradas en la lepra tuberculoide limítrofe o en las reacciones reversas. Se discute el significado de estos hallazgos en relación al uso de vacunas en la lepra.Leprosy is a chronic granulomatous disease involving the intracellular parasitization of the cells of the mononuclear phagocyte series (MPS) with Mycobacterium leprae.
Resistance to intracellular organisms is mediated by a cellular immune reaction involving macrophages and specifically sensitized T cells (4). Resistance is strongest in the polar tuberculoid and borderline tuberculoid forms of the clinical spectrum and is associated with the formation of epithelioid-cell granulomas containing infiltrating T lymphocytes and very few, or no, bacilli. In the lesions of lepromatous leprosy, at the opposite pole, resistance is low and granulomas contain foamy macrophages packed with large numbers of organisms (7). Clinical manifestations depend upon the strength of the patient's specific cell-mediated immune response to the infecting organism. This also accounts for reversal reactions observed along the spectrum which are.due to a rapid development of states of hypersensitivity (1,10).
A model in the guinea pig has been established in our department to study granuloma induction by mycobacteria (5). Intradermal injection of BCG vaccine into the ear induced a granuloma in the draining lymph node which peaked in 2 weeks and was characterized by epithelioid-cell infiltration comparable to granulomas in tuberculoid leprosy. At the ultrastructural level, the epithelioid-cell cytoplasm may contain stacked rough endoplasmic reticulum with few other organelles. The nucleus is pale with a prominent nucleolus. When cobaltirradiated M. leprae organisms were similarly injected, granuloma formation was observed after 5 weeks. The granulomas contained phagocytosing macrophages, and resembled those in lepromatous leprosy lesions. A subsequent study revealed that haptenization of Co-irradiated M. leprae with fluorescein isothiocyanate (FITC) led to a granulomatous response similar to that induced by BCG (11). It is possible that haptenization increased the antigenicity of M. leprae for specific T-cell activation such as occurs with live BCG organisms.
Vaccination with BCG or BCG plus M. leprae has been used in trials for the immunoprophylaxis of leprosy. It was the purpose of this study to see whether presensitization with BCG or M. leprae might in any way alter the granulomatous response to M. leprae, particularly, whether a macrophage-type granuloma might be converted partly into an epithelioid-cell granuloma by earlier exposure to a vaccine. Acid-fast bacilli (AFB) are rarely detectable in mycobacteria-induced granulomas in this guinea pig model, although the remains of phagocytosed organisms are seen in electron micrographs of granulomas formed in response to dead M. leprae (5). It is thus not possible to assess clearance rates of mycobacteria in response to vaccination, and our interest was focused on the histopathological evidence of hypersensitivity and tissue damage.
MATERIALS AND METHODS
Animals. Outbred Hartley strain female guinea pigs, weighing 250 g-300 g, were obtained from David Hall (Newchurch, Staffs, U.K.). They were fed on RGP pelleted diet, supplemented with cabbage.
Mycobacteria. Live Pasteur strain M. bovis BCG, as a suspension in saline, was provided by the Pasteur Institut, Paris, France (8). Cobalt-irradiated (2.5 Mrad), armadillo-derived M. leprae was provided by Dr. R. J. W. Rees of the National Institute for Medical Research, Mill Hill, London.
Presensitization. Guinea pigs were presensitized by injecting 1 x 107 live BCG or 1 x 109 cobalt-irradiated M. leprae intradermally into the right flank.
Induction of granuloma. Co-irradiated M. leprae organisms (1 x 109) were injected intradermally into the dorsum of the right ear 2 weeks after presensitization with BCG. Guinea pigs presensitized with M. leprae were similarly injected 5 weeks after presensitization. Control guinea pigs were injected with 1 x 109 cobalt-irradiated M. leprae intradermally into the right ear only.
Draining post-auricular lymph nodes were collected for study 2 weeks and 5 weeks after the injection of M. leprae into the ear for the induction of granulomas. Lymph nodes were weighed individually on a torsion balance, and a portion of each node was fixed in Carnoy's fluid, processed, and stained with hematoxylin and eosin (H&E) for lightmicroscopy. Samples for electron-microscopy were cut into small pieces and fixed in 4% glutaraldehyde containing 0.05 M cacodylate buffer, pH 7.4, for 24 hr at 4ºC. Samples were cut and processed as described elsewhere (6).
Histological study. Granuloma formation was noted by the presence of infiltration by cells of the mononuclear-phagocyte series. Areas of infiltration from random transverse sections were traced on white paper from a projection microscope image (× 60 magnification). Measurements were performed using a planimeter (1 rev = 100 cm2; constant = 18,728). The area of infiltration was expressed as a percentage of the total area of the section.
Statistical analysis. Where appropriate, results were compared by Student's t test.
RESULTS
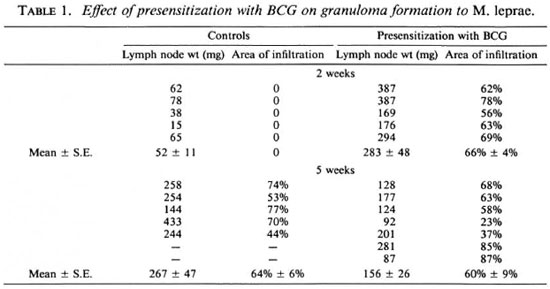
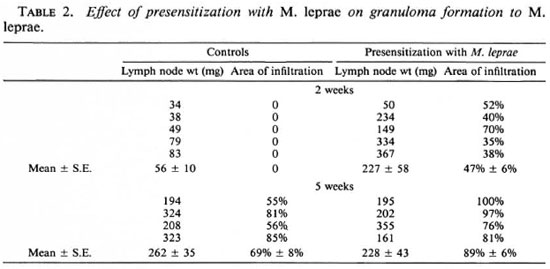
A granulomatous response to cobalt-irradiated M. leprae occurred at 2 weeks in animals presensitized with either BCG or M. leprae, whereas nonpresensitized guinea pigs did not show a response at this time (Tables 1 and 2). Histologically, cells of the mononuclear-phagocyte series (MPS) formed the infiltrates in all groups. In BCG-presensitized animals, the lymph node weights and areas of infiltration at 2 weeks were comparable to those in control animals at 5 weeks. By 5 weeks there was a significant (p < 0.05) decrease in lymph node weight, but there was considerable variation in weights and areas of infiltration. At this time, there was histological evidence of the presence of fibrosis. In M. Leprae-presensitized animals, the lymph node weights at 2 weeks were comparable to those in the control group at 5 weeks. The areas of infiltration, however, were less. The lymph node weights at 5 weeks were similar to those at 2 weeks, but there was a significant increase (p < 0.01) in infiltration. Histological examination revealed areas of necrosis.
Ultrastructure. Ultrastructural observations revealed morphologic heterogeneity of the MPS cells in the granulomatous infiltrates at 2 weeks after granuloma induction in BCG-presensitized animals. A majority of the cells had the appearance of epithelioid cells (5). They had round or oval nuclei with finely marginated heterochromatin and a prominent nucleolus. Rough endoplasmic reticulum (RER) was present as stacks or single strands. Golgi apparatus was distinct in many cells. Mitochondria and other cytoplasmic organelles were present in varying proportions in some of the cells. A frequently observed cell was one which contained RER as well as numerous, wellformed, mitochondria suggestive of early stages of epithelioid differentiation. Complex interdigitations between epithelioid cells with fimbriated borders were often seen (Fig. 1). Some cells showed a paucity of organelles, with scanty or no RER, and were suggestive of a lack of activity.
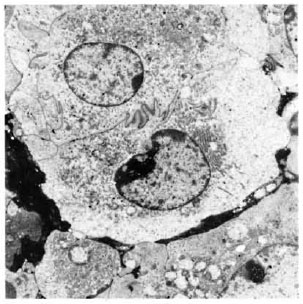
Fig. 1. Electronmicrograph of interdigitating epithelioid cells in 2-wk M. leprae granuloma in the lymphnode after BCG presensitization ( × 4500). Note palenucleus with prominent nucleolus and RER.
Phagocytosing cells with cytoplasmic extensions were present. The nuclei were round or indented and contained heterochromatin. The cytoplasm contained mitochondria and other organelles. The presence of endoplasmic reticulum with cisternal dilatations was also a feature in some of these cells (Fig. 2). In many areas, the cells formed a syncytium and giant cell formation resulted from the fusion of phagocytic cells.
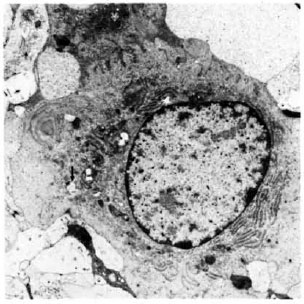
Fig. 2. Electronmicrograph of cell of mononuclearphagocyte series in 2-wk M. leprae granuloma in the lymph node after BCG presensitization (× 7500). Note RER and phagocytosis (↑).
Five weeks after granuloma induction in BCG-presensitized animals, infiltrates contained epithelioid-type cells. Cells of the MPS showing early epithelioid differentiation with RER and mitochondria were often observed (Fig. 3).Langhans' giant cells were occasionally present. A few of the cells showed vesicular cytoplasm. A noteworthy feature was the presence of collagen as fine strands or bundles in the intercellular matrix (Fig. 4). However, fibroblasts were not identified.
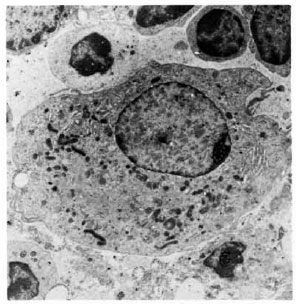
Fig. 3. Electronmicrograph of epithelioid cell in 5-wk M. leprae granuloma in the lymph node after BCG presensitization (× 4500). Note well-formed mitochondria and RER.
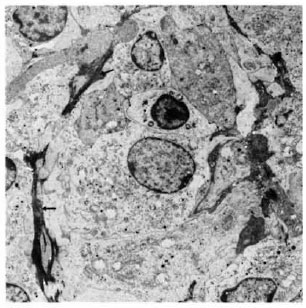
Fig. 4. Electronmicrograph showing infiltration with collagen formation (↑) in 5-wk M. leprae granuloma in the lymph node after BCG presensitization(× 3000). Note absence of typical fibroblasts.
In M. Leprae-presensitized animals the granulomas at 2 and 5 weeks contained mainly macrophages, and resembled those seen in unsensitized animals at 5 weeks. Phagocytosis was seen in some cells.- At 5 weeks there was evidence of cell degeneration and cell death as shown by the presence of swollen mitochondria, shrinking or disappearance of nuclei and dissolution of cell borders.
In the guinea pigs injected with M. leprae alone, without presensitization, most of the cells were macrophages as described previously (5). Phagocytosis was seen in some of these cells (Fig. 5).
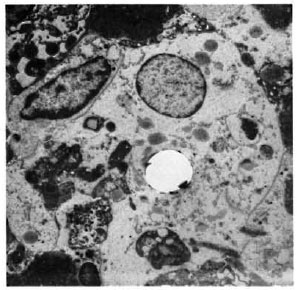
Fig. 5. Electronmicrograph of a phagocytic macrophage in a 5-wk M. leprae granuloma in the lymph node of a nonpresensitized animal (× 2500).
DISCUSSION
The present study shows that presensitization with BCG or cobalt-irradiated M. leprae has accelerated the onset of granuloma formation in the lymph node draining the site of the subsequent intradermal injection of M. leprae into the ear. No granuloma formation to M. leprae was found at 2 weeks in control animals that had not been presensitized, although granulomas were fully developed by 5 weeks. However, in presensitized animals, granulomas were found at 2 weeks, a similar time course to that of granuloma formation in response to BCG vaccine (5). At 2 weeks, the BCG-presensitized animals showed infiltration which consisted of an ultrastructurally heterogenous population of cells of the mononuclear phagocyte series (MPS). Langhans' giant cell formation and cells with complex interdigitations were present. Phagocytosing cells which also contained endoplasmic reticulum were observed. However, a majority of the MPS cells showed epithelioid differentiation of various stages. Epithelioid cells containing RER and well-developed mitochondria were frequently observed; these cells were also seen in the 5-week granulomas of the BCG-presensitized animals in which collagen formation was present. Phagocytic cells containing M. leprae, but with abundant endoplasmic reticulum, have been described in a Mitsuda granulomatous hypersensitivity reaction in borderline tuberculoid (BT) leprosy (5). In the same study, epithelioid cells containing RER as well as mitochondria were described in two cases of BT leprosy in reaction.
The type of epithelioid cell which has a rough endoplasmic reticulum and is nonphagocytic is thought to be a secretory cell(13). Its development at various stages may depend upon the nature of the antigenic stimulus. Healing is a feature of epithelioid granulomas, but it is not established whether the epithelioid cell has a role in promoting fibrogenesis (8). It is possible that acute exacerbations followed by residual nerve damage may arise due to the development of the epithelioid cells following further stimulation by M. leprae antigens in clinical reversal reactions. Studies of the lymphocyte function revealed a correlation with hypersensitivity reactions that would lead to reversal reactions (1).
Granulomas in M. Leprae-presensitized animals were not of the hypersensitivity type. They contained macrophages, but epithelioid cells were rarely seen. At 5 weeks, cell degeneration and cell death were observed both by light- and electron-microscopy. Cell necrosis as a result of presensitization in a model where there is little evidence of cell-mediated immune activity could be due to an antibody-mediated event, or could be due to a local accumulation of tumor necrosis factor (TNFα).
Protection against M. leprae is considered to be a cell-mediated immune mechanism. Macrophage interactions with specifically elicited T cells are readily demonstrated in vitro and well perceived at the histopathological level. Vaccination has been proposed as a means of mounting protection against M. leprae (2). A number of recently initiated trials in northern Malawi, Venezuela, and India have been designed using a combination of BCG with killed M. leprae and comparing this with BCG alone. The evaluation of the efficacy of a vaccine is ultimately the absence of clinical disease. Moreover, it is observed that vaccination may not achieve uniform results as far as individuals at risk are concerned and in different endemic areas. Environmental and genetic factors and prevalent forms of the disease in a defined geographic area may influence the overall outcome (3). The results of immunotherapy in Venezuela, using a combination of BCG and killed M. leprae, are also based on similar factors (2). However, there is a suggestion that vaccines might stimulate a state of hypersensitivity with or without producing a positive stage of protective immunity against M. leprae (9). In this situation, vaccination could make an individual more prone to develop a reversal reaction. It appears from these studies that BCG vaccination might accelerate granuloma formation and produce a tuberculoid type of granuloma in circumstances where one would normally expect to find a lepromatous lesion.
In conclusion, BCG presensitization induced changes in granulomatous responses to M. leprae in the guinea pig comparable to reversal reactions in human leprosy. The effect of presensitization with BCG is probably to increase the strength of the cell-mediated immune response to M. leprae. The effect may be comparable to that obtained by linking a hapten to the surface of M. leprae (11). There is, no doubt, an increase in the strength of the cell-mediated hypersensitivity granulomatous reaction. Evidence for the protective effect of the cellmediated protective immune response has been available only in a limited number of trials in man (3), and these have involved mainly BCG vaccine alone. Care, therefore, should be taken to avoid boosting the potential for the development of reversal reactions without more evidence of a considerable protective function.
Acknowledgments. This work was supported by a grant from the British Leprosy Relief Association (LEPRA). The authors wish to thank Panayiotis Papasavva and Francis Schindler for their technical assistance and June Saxby for typing the manuscript.
REFERENCES
1. BJUNE, G., BARNETSON, R. ST.C , RIDLEY, D. S. and KRONVALL , G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of lesions. Clin. Exp. Immunol. 25(1976)85-94.
2. CONVIT, J., ULRICH, M., ARANZAZU, N., CASTELLANOS, P. L., PIRANDI, M. E. and REYES, O. The development of a vaccination model using two microorganisms and its application in leprosy and leishmaniasis. Lepr. Rev. 57(1986)263-273.
3. FINE, P. E. M. and PONNIGHAUS, J. M. Leprosy in Malawi. 2. Background design and prospects of the Karonga Prevention Trial, a leprosy vaccine trial in northern Malawi. Trans. R. Soc. Trop. Med. Hyg. 82(1988)810-817.
4. MACKANESS, G. B . and BLANDEN, R. V. Cellular Immunity. Progr. Allergy 11(1967)89-140(194 réf.).
5 . NARAYANAN, R. B., BADENOCH-JONES, P. and TURK, J. L. Experimental mycobacterial granulomas in guinea pig lymph nodes: ultrastructural observations. J. Pathol. 134(1981)253-265.
6 . RIDLEY, M. J., BADENOCH-JONES, P. and TURK, J. L. Ultrastructurc of cells of the mononuclearphagocyte scries (MPS) across the leprosy spectrum. J Pathol. 130(1980)223-227.
7. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
8. TURK, J. L. Immunologic and non-immunologic activation of macrophages. J. Invest. Dermatol. 74(1980)301-306.
9. TURK, J . L. Dissociation between allergy and immunity in mycobacterial infections. Lepr. Rev. 54(1983)1-8.
10. TURK, J. L. and BRYCESON, A. D. M. Immunological phenomena in leprosy and related diseases. Adv. Immunol. 13(1971)209-266 (214 ref.).
11. VERGHESE, S., CURTIS, J . and TURK, J. L. Epithelioid cell granuloma induction in the guinea pig by haptenated Mycobacterium leprae. Cell. Immunol. 107(1987)307-316.
12. WANSTRUP, J . and CHRISTENSEN, H. E. Sarcoidosis. 1. Ultrastructural investigations on epithelioid cell granulomas. Acta Pathol. Microbiol. Scand. 66(1966)169-185.
13. WILLIAMS, W. J., JAMES, E. M., ERASMUS, D. A. and DAVIES, T. The fine structure of sarcoid and tuberculous granulomas. Postgrad. Med. J. 46(1970)496-500.
1. M.D., Department of Pathology, Royal College of Surgeons of England, 35/43 Lincoln's Inn Fields, London WC2A 3PN, U.K.
2. B.Sc, Department of Pathology, Royal College of Surgeons of England, 35/43 Lincoln's Inn Fields, London WC2A 3PN, U.K.
3. Ph.D., Department of Pathology, Royal College of Surgeons of England, 35/43 Lincoln's Inn Fields, London WC2A 3PN, U.K.
Reprint requests to Dr. Jill Curtis.
Received for publication on 15 February 1990.
Accepted for publication in revised form on 18 May 1990.