- Volume 58 , Number 4
- Page: 690–6
Effect of simultaneous administration of lnterferon-γ and chemotherapy against Mycobacterium leprae in experimental infection in nude mice
ABSTRACT
The possibility of synergy between immunotherapy with recombinant interferongamma (IFN-γ) and chemotherapy with rifampin (RMP) and dapsone (DDS) against Mycobacterium leprae was examined in nude mice. IFN-γ alone failed to show any effect on the growth of M. leprae in the nude mouse foot pad. No synergy was demonstrable between DDS, cither at 0.0001 % or at 0.001 %, and IFN-γ. A subinhibitory level of RMP with IFN-γ was also ineffective, but RMP at 0.006% with IFN-γ produced a statistically significant enhancement of killing (26-fold) when compared with RMP at 0.006% only. It should be emphasized, however, that results obtained in the immunodeficicnt nude mouse model may not be comparable to those which might have been given by lepromatous leprosy patients.RÉSUMÉ
La possibilité de synergie entre un recombinam interferon-gamma (IFN-γ) et la chimiothérapie à Ia rifampicine (RMP) et la dapsone (DDS) vis-à-vis de Mycobacterium leprae a été examinée chez la souris nue. L'IFN-γ seul ne montra aucun effet sur la croissance de M. leprae dans le coussinet plantaire de la souris nue. Aucune synergie n'a pu être démontrée entre la DDS, que ce soit à 0.0001% ou à 0.001%, et l'IFN-γ. Une concentration subinhibitrice de RMP avec l'IFN-γ, n'était pas efficace non plus, mais la rifampicine à 0.006% jointe à l'IFN-γ produisit un renforcement statistiquement significatif de l'effet bactéricide (26 fois), comparé à celui de la rifampicine à 0.006% seule. Il devrait être souligné cependant que les résultats obtenus chez la souris nue immunodéficiente peuvent ne pas être comparables à ceux qui pourraient être observés chez, des patients lépromateux.RESUMEN
Usando el modelo del ratón desnudo, se examinó la posibilidad de sinergismo entre la inmunoterapia con interferon gamma recombinante (IFN-γ) y la quimioterapia con rifampina (RMP) y dapsona (DDS) contra el Mycobacterium leprae. El IFN-γ sólo no mostro nin-gún cfecto sobre el crecimiento del M. leprae en la almohadilla plantar del ratón. No se observo sinergismo entre el DDS (al 0.0001% o al 0.001%) y el IFN-γ. Tampoco fuc efectivo un nivel subinhibitorio de la RMPasociado al IFN-γ. Sin embargo, cn comparación con la RMP sola, la RMP al 0.006% asociada al IFN-γ produjo un incremento significativo (de 26 veces) en su capacidad bactericida. No obstante lo anterior, los resultados obtenidos en el ratón desnudo inmunodeficientc, podrían no ser comparables con los obtenidos en los pacientes lepromatosos.Currently, it is recognized that, for efficient chemotherapy of leprosy, a treatment regimen must ensure that the emergence of a drug-resistant variant of the infecting strain of Mycobacterium leprae (manifested by clinical relapse under treatment) must be prevented, and that the bacterial population suffers a satisfactory rate of killing, for which at least one bactericidal drug must be included in the regimen. These specifications are now generally satisfied by the simultaneous administration of dapsone (cheap, bacteriostatic and only feebly bactericidal), rifampin (the most bactericidal of the antileprosy drugs), and either clofazimine or prothionamide (both with bactericidal activity). This combination is highly effective in preventing the emergence of a resistant mutant, and recent reviews (23) indicate that its administration for 2 years appears to reduce the number of viable M. leprae in the patient's body below the level at which regrowth can occur after cessation of treatment (post-treatment clinical relapse).
The patient's cell-mediated immune mechanisms play a part (if rather a small one) in the bactericidal process, and if these could be stimulated to a higher level of activity, drug dosage or period of administration might be reduced without loss of efficacy and with a reduction of side effects. Immunostimulatory reagents have therefore been sought. Chemical immunostimulants have posed problems, especially as to toxicity. Biological immunostimulants with low toxicity have been used in a variety of situations, e.g., the use of BCG as an immunotherapy in bladder cancer (3,13) the attempted suppression of growth of leprosy bacilli by a vaccine containing M. leprae and BCG (5,6), or by vaccines containing other mycobacterial antigens, such as the ICRC bacillus (7), or M. vaccae (1). There has been development of a newly identified lymphokine, interferon-gamma (IFN-γ), leading to the isolation of a recombinant clone and large-scale production of this substance. It has been used in attempts to modify the immune state of individuals, for example, in the acquired immunodeficiency syndrome (22). On the basis of current evidence, it appears that IFN-γ is the major regulator of macrophage antimicrobial mechanism (17,19,20). The antimicrobial mechanism is primarily based on oxygen-dependent killing or inhibitory activity (19,21) but oxygen-independent mechanisms have also been demonstrated (4,24). IFN-γ has been shown to cause enhanced killing of a number of intracellular parasites such as toxoplasma (21), leishmania (18), listeria (11), and a number of other similar parasites (25). However, there was conflicting evidence of its activity against mycobacteria (2,8,12,16) .
In lepromatous leprosy, leprosy bacilli are found in abundance within macrophages. Presumably due to the lack of an adequate immunological stimulus for the patient's T lymphocytes, these macrophages fail to be activated to the extent needed to produce killing of the intracellular bacilli, although more of the bacilli are dead than alive. Defective production of IFN-γ has been reported both intracellularly and in the lesions (20,33). It is therefore possible that IFN-γ, given alone or in combination with antileprosy agents, may be able to make these macrophages functionally more active, resulting in enhanced bactericidal action. This study was thus designed to examine whether enhanced bactericidal activity could be demonstrated experimentally with different combinations of antibacterial agents plus IFN-γ regimens in parallel with IFN-γ only.
MATERIALS AND METHODS
Animals. Nude mice allow extensive growth of M. leprae, leading to a lepromatous type of disease in these animals (14). Therefore, nude mice were used in this study to allow examination of the effect of IFN-γ without the complicating effect of functional T lymphocytes, since they are absent in these mice. Nude mice of an outbred strain (CD1), with a microbiologically defined gut flora, obtained from a commercial source (Charles River), were maintained in an otherwise germ-free condition in isolators.
Inoculum. Human leproma-derived M. leprae maintained in nude mice were used as the inoculum; they had previously been found to be sensitive to both dapsone (DDS) and rifampin (RMP). The acid-fast bacilli (AFB) in a foot-pad homogenate from an infected mouse were counted microscopically after Ziehl-Neelsen staining and diluted to provide a suspension containing 5 × 104 AFB/ml. Twenty µl of the suspension was inoculated into each hind foot pad of these mice (104/foot pad).
Experimental protocol. Thirty days after inoculation the mice were divided into eight treatment groups and a control group, and the treatments were started. Each treatment was continued for 60 days, following Shepard's kinetic technique (28). Control mice, and those that had received IFN-γ only, were harvested at the end of the treatment period (3 months after inoculation) and every month thereafter, and counts were made of the AFB/foot pad. When the counts in control mice indicated that growth had occurred (106 AFB/foot pad), three mice were harvested from each treated group every month, the foot pads were homogenized, and the AFB counts were determined.
By the kinetic technique, if growth in a treated group of mice is delayed, compared with that in the controls, bacteriostasis has occurred. If the delay is longer than the period of drug administration, bactericidal action is assumed to have occurred if, as in this case, the drugs used are rapidly cleared after cessation of treatment.
Drug treatment. RMP was mixed with a powdered diet which was then made up into small biscuits (about 3 cm x 2 cm x 1 cm) and sterilized by irradiation. DDS was mixed with sterile drinking water, freshly prepared every day. In a pilot experiment, the DDS concentration in mouse serum after oral administration of the drug in drinking water was determined. These estimations were carried out by Professor Seydel at the Research Institute for Biological Sciences, Borstel, West Germany. The drug was administered for 8 weeks at a dose of 0.001 % in water, and samples were taken at 1, 4, and 8 weeks. The levels achieved were between 0.124 and 0.159 µg/ml (0.0001% dapsone in diet produces a level of 0.01 µg/ml 9). Recombinant IFN-γ, produced by Genentech Inc., U.S.A., and obtained as a gift through Boehringer International, Vienna, was confirmed to be potent by a bioassay test using mouse L cells and EMC virus, both by the suppliers and by the laboratory of Dr. Alan Morris at Warwick University, Coventry, U.K. It was injected intraperitoneally twice weekly at a dose of 1000 units during the 60-day treatment period. The results were statistically analyzed using a twofactor analysis of variance.
RESULTS
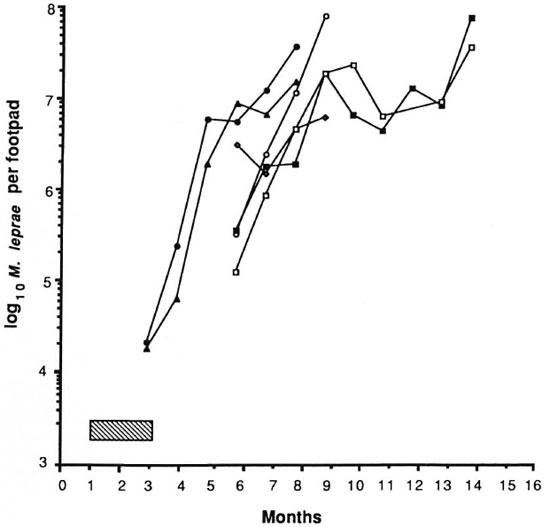
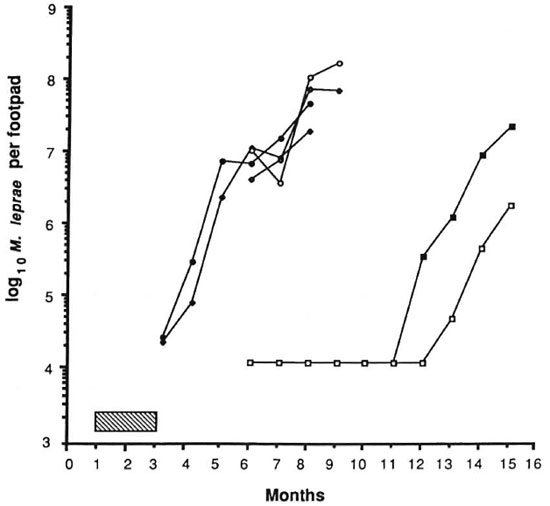
The results of the experiment are shown in Figures 1 and 2. The control mice showed exponential growth of M. leprae from 3 months after inoculation and reached 108 AFB/foot pad at 8 months. IFN-γ given alone at a dose of 1000 units twice weekly failed to produce any appreciable delay in growth of the organisms in comparison to control counts. DDS at 0.0001%, given either alone or in combination with IFN-γ, and IFN-γ with DDS at 0.001% produced growth delays of less than or equal to the period of drug administration, indicating only bacteriostasis. A subinhibitory dosage of RMP (0.006%), alone or in combination with IFN-γ, did not have any effect on the growth of M. leprae. In both of these groups, foot pad counts reached a peak of about 108 at the same time and no growth delay was demonstrable (Fig. 2). As expected, RMP at 0.006% demonstrated bactericidal action by producing a long growth delay; growth became manifest 9 months after the drug had been stopped. The addition of IFN-γ to this regimen produced a further growth delay of over 45 days. Statistical analysis, using the two-factor analysis of variance, showed that the delay was highly significant (F 55.6, p < 0.001). The difference in the counts between the treatment groups (RMP vs RMP + IFN-γ) was 26 times. These results seem to indicate that IFN-γ fails to enhance the effect of a bacteriostatic drug such as DDS, but with RMP at a therapeutic dose a significant excess kill was effected.
Fig. 1. Effect of treatment with IFN-γ and dapsone on growth of M. leprae in nude mouse foot pads. Hatched area indicates period of treatment (60 days); each point represents average count in 3 foot pads;  = controls;
= controls;  = IFN-γ;
= IFN-γ; 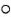 = 0.0001% DDS;
= 0.0001% DDS; 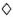 = 0.0001% DDS + IFN-γ;
= 0.0001% DDS + IFN-γ;  = 0.001% DDS;
= 0.001% DDS;  = 0.001% DDS + IFN-γ.
= 0.001% DDS + IFN-γ.
Fig. 2. Effect of treatment with IFN-γ and rifampin on growth of M. leprae in nude mouse foot pads. Hatched area indicates period of treatment (60 days); each point represents average count in 3 foot pads;  = controls;
= controls; 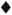 = IFN-γ;
= IFN-γ; 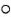 = 0.0006% RMP;
= 0.0006% RMP; 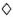 = 0.0006% RMP ± IFN-γ;
= 0.0006% RMP ± IFN-γ;  = 0.006% RMP;
= 0.006% RMP;  = 0.006% RMP + IFN-γ.
= 0.006% RMP + IFN-γ.
DISCUSSION
The outcome of an infection during treatment is determined by a) the effectiveness of the antimicrobial used and b) the competence of the immune system. A competent immune system usually works hand in hand with the antibiotic, resulting in a synergistic suppression of growth with microbial kill (32). The most common expression of this synergy is when microbial destruction by the immune system follows the inhibition of microbial growth by an antibiotic. If, on the other hand, the immune system is incapable of playing its supplementary destructive role, the antibiotic is left alone to perform this function which, in the majority of cases, it fails to execute fully. Lepromatous leprosy is an example of the kind of disease in which the immune system is incapable of dealing adequately with the invading organism and, therefore, the antimicrobial is effectively left to kill the bacterial population almost alone. This is particularly evident during treatment of leprosy by a drug such as DDS. In fact, many years of DDS treatment may still leave DDSsensitive viable bacilli ("persisters") which may cause a relapse. Persisters are also found after prolonged multiple-drug therapy, but might be eliminated if there were a more powerful immune response.
Immunostimulants have previously been used in leprosy research. For example, levamisole was used by some workers in attempts to activate the cell-mediated immune system (15,29,30) However, these studies failed to show any effect.
IFN-γ, a lymphokine produced by T lymphocytes, has been shown by some workers to activate macrophages, resulting in the production of oxidative bactericidal products such as hydrogen peroxide and superoxide anions (21,23) . Others, however, showed that oxidative intermediates are produced only when the macrophages are in an induced state of activation (27). Although the results are equivocal (31), there is a trend of thought that IFN-γ may act as a macrophage stimulant, and may have an important role in the suppression or killing of M. leprae. In order to test this theory, IFN-γ was injected into leproma sites, producing an increased cellular response and a reduction in the number of bacilli (10,20,26,33). However, since over 70% of M. leprae detected by acid-fast staining in the tissues are dead, this may signify an enhanced removal of dead bacilli rather than enhanced killing. The lack of an inhibitory effect of IFN-γ on its own was also reported by other workers in in vitro studies. It failed to inhibit growth of M. microti in mouse peritoneal macrophage cultures (12), while it apparently enhanced the growth of M. tuberculosis (8) and M. lepraemurium (16). Nude mice are devoid of T lymphocytes and, hence, are unable to mount a cell-mediated response. In the work reported here, a combination of IFN-γ witheithera minimal inhibitory concentration (0.0001%) of DDS in the diet or 10 times that amount (0.001%) failed to show any synergistic effect; i.e., there was no synergy with a bacteriostatic drug even at a fully inhibitory level. In an in vitro experimental system with other mycobacteria (12), isoniazid and RMP, both actively bactericidal drugs, failed to synergizc with IFN-γ against M. tuberculosis in mouse peritoneal macrophage cultures. However, in our work an enhanced bactericidal effect was produced by the addition of IFN-γ to the therapeutic dose of RMP (0.006%). The treatment schedules tried in this study included those intended to discover whether subinhibitory concentrations of an antibiotic in combination with IFN-γ would achieve synergy of bactericidal action. This did not happen with 0.0006% RMP, which suggests that synergy is possible only at a therapeutic dose of a drug.
The finding of synergy between such a level of RMP and IFN-γ is encouraging. The present results need further evaluation by more detailed studies with variation of the IFN-γ regimens, including variation in time and dosage.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. The authors are grateful to Miss Margaret Nicol for excellent technical assistance and to professor G. R. F. Hilson for many helpful suggestions.
REFERENCES
1. BAHR, G. M., STANFORD, J. L., ROOK, G. A. W., REES, R. J. W., ABDELNOOR, A. M. and FRYHA, G. J. Two potential improvements to BCG and their effect on skin test reactivity in Lebanon. Tubercle 67(1986)205-218.
2. BANERJEE , D. K., SHARP, A. K. and LOWRIE , D. B. The effect of gamma-interferon during Mycobacterium (BCG) infection in athymic and euthymic mice. Microb. Pathog. 1(1986)221-224.
3. BROSMAN, S. A. The use of bacillus Calmette-Guerin in the therapy of bladder carcinoma in situ. J. Urol. 134(1985)36-39.
4. CATTERALL, J. R., BLACK, C. M., LEVENTHAL, J. P., RIZK, N. W., WACHTEL, U. S. and REMINGTON, J. S. Non-oxiative microbicidal activity in normal human alveolar and peritoneal macrophages. Infect. Immun. 55(1987)1635-1640.
5. CONVIT, J., ARANZAZU , N., ULRICH, M., ZUNIGA, M., DE ARAGON, M. E., ALVARADO, J. and REYES, O. Investigations related to the development of a leprosy vaccine. Int. J. Lepr. 51(1983)531-539.
6. CONVIT, J., ULRICH, M. and ARANZAZU, N. Vaccination in leprosy-observations and interpretations. Int. J. Lepr. 48(1980)62-65.
7. DEO, M. G., BAPAT, C. V., BHALERAO, V., CHATURVEDI, R. M., CHULAWALA, R. G. and BHATKI, W. B. Antileprosy potentials of the ICRC vaccine: a study in patients and healthy volunteers. Int. J. Lepr. 51(1983)540-549.
8. DOUVAS, G. S., LOOKER , D. L., VATTER, A. E. and CROWLE , A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophagc-associated mycobacteria. Infect. Immun. 50(1985)1-8.
9. ELLARD , G. A. Growing points in leprosy research. (4) Recent advances in the chemotherapy of leprosy. Lepr. Rev. 45(1974)31-40.
10. KAPLAN, G., NUSRAT, A., SARNO, E. N., JOB, C. K., MCELRATH , J., PORTO , J. A., NATHAN, C. F. and COHN, Z. A. Cellular responses to the intradermal injection of recombinant human γ-interferon in lepromatous leprosy patients. Am. J. Pathol. 128(1987)345-353.
11. KIDERLEN, A. F., KAUFMANN, S. H. E. and LOHMANN-MATTHES, M. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur. J. Immunol. 14(1984)964-967.
12. KHOR, M., LOWRIE, D. B. and MITCHISON , D. A. Effects of recombinant interferon-gamma and chemotherapy with isoniazid and rifampicin on infections of mouse peritoneal macrophages with Listeria monocytogenes and Mycobacterium microti in vitro. Br. J . Exp. Pathol. 67(1986)707- 717.
13. LAMM , D. L. Bacillus Calmette-Gucrin immunotherapy for bladder cancer. J. Urol. 134(1985)40-47.
14. LANCASTER, R. D., HILSON, G. R. F., MCDOUGALL, A. C. and COLSTON, M. J. Mycobacterium leprae infection in nude mice: bacteriological and histological responses to primary' infection and large inocula. Infect. Immun. 39(1983)865-872.
15. MARTINEZ, D. and ZAIAS, N. Levamisolc as adjunct to dapsonc in leprosy. (Letter) Lancet 2(1976)209-210.
16. Mor, N., Goren, M. B. and CROWLE, A. J. Enhancement of growth of Mycobacterium lepraemurium in macrophages by gamma interferon. Infect. Immun. 57(1989)2586-2587.
17. MURRAY, H. W. Intcrferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann. Intern. Med. 108(1989)595-608.
18. Murray, H. W., and Rubin, B. Y. and ROTHERMAL, C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-γ is the activating lymphokine. J. Clin. Invest. 72(1983)1506-1510.
19. NATHAN, C. F. Interferon-gamma and macrophage activation in cell-mediated immunity. In: Mechanisms of Host Resistance to Infectious Agents. Tumors and Allografts. Steinman, R. M. and North R. J., eds. New York: Rockefeller University Press, 1986, pp. 165-184.
20. NATHAN, C. F., KAPLAN, G., LEVIS , W. R., NUSRAT, A., WITMER, M. D., SHERWIN, S. A., JOB, C. K., HOROWITZ, C. R., STEINMAN, R. M. and COHN , Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
21. NATHAN, C. F., MURRAY, H. W., WIEBE, M. E. and RUBINB. Y. Identification of intcrferon-7 as the lymphokine that activates human macrophages oxidative metabolism and antimicrobial activity. J. Exp. Med. 158(1983)670-689.
22. PARKIN, J. M., EALES, L. J., G ALAZKA, A. R. and P INCHING, A. J. Atopic manifestations in the acquired immune deficiency syndrome: response to recombinant interferon gamma. Br. Med. J. 294 (1987) 1 185-1 186.
23. REPORT OF PRE-CONGRESS WORKSHOP COMMITTEE, WORKSHOP 2. Chemotherapy, clinical and experimental. International Leprosy Congress, The Hague, 1988. Int. J. Lepr. 57 Suppl. (1989)278-279.
24. ROTHERMAL, C. D., RUBIN, B. Y., JAFFE, E. A. and MURRAY, H. W. Oxygen-independent inhibition of intracellular Chlamydia psittace growth by human monocytes and interferon-γ activated macrophages. J. Immunol. 37(1986)689-692.
25. ROTHERMEL, C. D., RUBIN, B. Y. and MURRAY,H. W. Gamma interferon is the factor in lym-phokine that activates human macrophages to in-hibit intracellular Chlamydia psittaci replication. J. Immunol. 131(1983)2542-2544.
26. SAMUEL, N. M., GRANGE, J. M., SAMUEL, S., LUCAS,S., OWILLI, 0. M., ADALLA, S., LEIGH, I. M. andNAVARRETTE, C. A study of the effects of intra-dermal administration of recombinant gamma in-terferon in lepromatous leprosy patients. Lepr. Rev.58(1987)389-400.
27. SHARP, A. K. and BANERJEE, D. K. Effect of gamma interferon on hydrogen peroxide productionby cultured mouse peritoneal macrophages. Infect.Immun. 54(1986)597-599.
28. SHEPARD, C. C. A kinetic method of study of activity of drugs against Mycobacterium leprae inmice. Int. J. Lepr. 35(1967)429-435.
29. SHEPARD, C. C., VAN LANDINGHAM, R. and WALK-ER, L. L. Effect of levamisole on Mycobacterium leprae in mice. Infect. Immun. 16 (1977)564-567.
30. SHER, R., WADEE, A. A., JOFFE, M., KOK, S. H., IMKAMP, F. M. J. H. and SIMSON, I. W. The in vivo and in vitro effects of levamisole in patients with lepromatous leprosy. Int. J. Lepr. 49(1981)159-166.
31. SIBLEY, L. D. and KRAHENBUHL, J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium terrae. Infect. Immun. 56(1988)1912-1919.
32. VAN DEN BROEK, P. J. Antimicrobial drugs, microorganisms, and phagocytes. Rev. Infect. Dis. 11(1989)213-245(387 refs.).
33. VOLC-PLATZER, B., STEMBERGER, H., LUGER, T., RADASZKIEWICZ, T. and WIEDERMANN, G. Defective intralesional intcrfcron-gamma activity in patients with lepromatous leprosy. Clin. Exp. Immunol. 71 (1988) 235-240.
34. WALKER, L. and LOWRIE, D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature 293(1981)69-71.
1. M.D., Ph.D., F.R.C.Path., Department of Medical Microbiology, St. George's Hospital Medical School, London SW17 ORE, U.K.
2. Ph.D., Department of Medical Microbiology, St. George's Hospital Medical School, London SW17 ORE, U.K.
Received for publication on 5 January 1990.
Accepted for publication in revised form on 15 May 1990.