- Volume 58 , Number 4
- Page: 704–16
The Chemotherapy of Leprosy. Part 1*
Editorial opinions expressed are those of the writers.
Worldwide use of the two multidrug regimens recommended by the World Health Organization (WHO) for the treatment of paucibacillary and multibacillary patients 1 has unequivocally demonstrated that they are currently the drug treatments of choice. Although THELEP (the Subcommittee on Clinical Trials of the Chemotherapy of Leprosy Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases) have recently initiated studies to explore potential ways of strengthening multidrug treatment, the results of these trials will not be available for at least 5 years. It is therefore an appropriate time to review the chemotherapy of leprosy. The historical developments that led to the introduction of multidrug treatment are described first, together with a consideration of the antibacterial activity and pharmacology of the three most important antileprosy drugs-dapsone, rifampin and clofazimine-;and methods to measure their concentrations. The problems posed to the treatment and control of leprosy by the selection and spread of dapsone-resistant strains of Mycobacterium leprae, by the phenomenon of drugsusceptible persisting bacilli ("persisters"), and by widespread deficiencies in patient compliance will then be considered. Finally, after a discussion of the rationale for multidrug treatment with combinations of dapsone, rifampin, and clofazimine, the characteristics of other potential antileprosy drugs are reviewed, and suggestions are made as to how they might be used to strengthen multidrug therapy in the future.
Leprosy control. Leprosy is still one of the major diseases afflicting the inhabitants of Asia and Africa. Throughout the world there are probably about 11 million cases 2,3 and prior to the introduction of multidrug treatment, their numbers were not decreasing to any significant extent. About one third have significant deformities. It is clear that for at least the next decade all hopes of controlling the disease must depend on improved case finding and treatment. As in the case of tuberculosis,4,5 it is likely that the effective treatment of infectious, highly bacilliferous patients is a far more efficient method of controlling the spread of the two diseases than mass vaccination. Thus, as in tuberculosis where smear-positive cases are very uncommon in children, multibacillary cases less than 15 years old are very rarely encountered in leprosy.6,7 As a consequence, even vaccination at birth with a vaccine that protects for 10-15 years will not significantly reduce the pool of transmitted infection. By contrast, treatment with rifampin-containing regimens almost certainly leads to a dramatic reduction in infectiousness within a few days.
This review focuses almost exclusively on the treatment of multibacillary patients, even though they form only a minority of all leprosy cases, firstly because such patients constitute the major source of transmission of the disease and secondly, because their huge bacterial populations have the potential for allowing the selection of drug-resistant strains that could eventually undermine the efficacy of available treatment and thus seriously impair hopes of controlling the disease.
Historical advances in the chemotherapy of leprosy
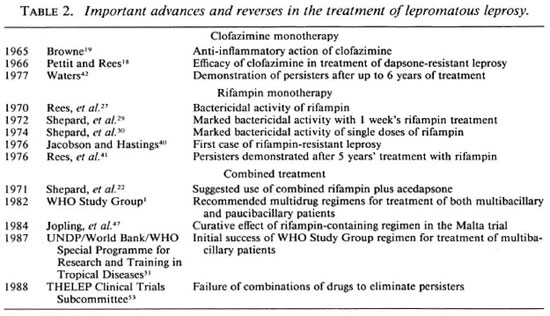
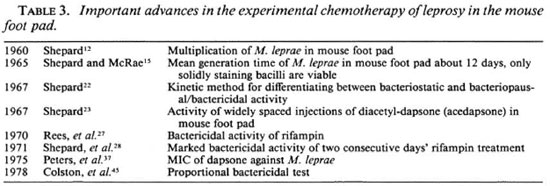
1943-1969: Dapsone and clofazimine; bacterial and morphological indices; the mouse foot-pad technique. Some of the most important advances and reverses in the chemotherapy of leprosy are summarized in Tables 1 and 2. Important advances in the experimental chemotherapy of leprosy in the mouse foot pad and in monitoring therapeutic progress in multibacillary patients are set out in Tables 3 and 4, respectively.
The first conclusive evidence of the activity of a drug in the clinical treatment of leprosy was the report in 1943 of Faget and his colleagues from Carville in the U.S.A. on Promin, a disubstituted derivative of dapsone (DDS).8 Previously, dapsone had been shown to be active against M. tuberculosis both in vitro and in vivo, and a number of disubstituted derivatives had then been synthesized for clinical use which were better tolerated when given on a gram scale than dapsone. However, over the next decade, evidence was obtained in both experimental animals and man that the activity of the disubstituted sulfones was probably due to their breakdown to dapsone in the body. This provided the rationale for the first clinical use of oral dapsone in the treatment of leprosy by Lowe and Smith working in Nigeria in 1949.9 The marked clinical response of patients to this well-tolerated and cheap drug was hailed as a miracle and over the next decade dapsone treatment was introduced throughout the world. Treatment with dapsone alone seemed to be highly successful in contrast to the experience in the first monotherapy trials in pulmonary tuberculosis, when after an initial favorable response to streptomycin or isoniazid treatment, patients relapsed through the selection of drug-resistant mutant strains.
It was soon appreciated that in order to compare the therapeutic activity of different drug regimens it was essential to monitor therapeutic progress quantitatively, and that the most promising approach would be to measure the rate of disappearance of leprosy bacilli from skin lesions during treatment. These endeavors culminated in the development of the "bacterial index" (BI) by Ridley in 1964 10 for quantifying M. leprae in skin smears on a logarithmic scale. However, it gradually became clear that the removal of dead leprosy bacilli from the tissues of lepromatous patients was a very slow process, and that a more sensitive and rapid method of assessing therapeutic response would be to estimate the rate of decline in the proportion of solid-staining, morphologically intact and therefore presumably viable, bacilli as described by Waters and Rees 11 in 1962.
The key discovery, which was to revolutionize the chemotherapy of leprosy, was that of Shepard in 1960.12 He showed that the inoculation of small numbers (5000-10,000) of M. leprae from the skin lesions or nasal washings of untreated lepromatous patients into the foot pads of normal mice was followed by limited multiplication to a ceiling of about 104 bacilli within a period of about 6 months. The mouse foot-pad model was soon employed to show that the activity of an established antileprosy drug, such as dapsone, in preventing the multiplication ofM. leprae could readily be demonstrated experimentally13 and, by the same token, that the potential activity of other previously unproven compounds could be explored.
By 1964, Pettit and Rees 14 had used the mouse foot-pad model to demonstrate the existence of dapsone-resistant strains of M. leprae, although they estimated that the first three patients who had relapsed with such strains had been selected from a total population of about 5000 and that the vast majority appeared to have been successfully treated with dapsone monotherapy. This study incidentally provided the first proof of chronic poor compliance with oral dapsone treatment since Pettit and Rees identified four patients with drug-susceptible organisms who had appeared not to respond to many years of supposedly well-supervised treatment, but who responded promptly when dapsone was given by injection.
In 1965, Shepard and McRae 15 confirmed that only solid-staining bacilli were viable, and estimated the mean generation time of M. leprae in the mouse foot pad to be about 12 days, a finding subsequently confirmed by other workers.16-17 Clofazimine was first used to treat patients with proven dapsoneresistant leprosy by Pettit and Rees in 1966,18 therapeutic response being measured by the fall in the morphological index (MI). In the previous year, Browne 19 noticed that in addition to its direct antibacterial action, clofazimine had significant anti-inflammatory action. This was to prove of considerable value for the treatment of erythema nodosum leprosum (ENL, or type 2 reactions).
In 1966, Ridley and Jopling 20 described a five-point histological/immunological classification of leprosy which was to become the key determinant in selecting patients for controlled clinical trials and in understanding the immunology of leprosy. In the same year, Rees 21 obtained enhanced multiplication of M. leprae in thymectomized/irradiated mice, demonstrating that in the mouse, as in man, the major determinant limiting bacterial multiplication was the extent of cell-mediated immunity.
In the following year, Shepard 22 described his "kinetic" method in which drugs were administered for a limited period (usually about 60 days) during the logarithmic phase of growth, to determine whether they possessed solely bacteriostatic or bacteriopausal/bactericidal activity, characteristics that were soon recognized as being of enormous clinical relevance. In the same year, Shepard 23 demonstrated that widely spaced injections of a slow-release dapsone formulation (diacetyl-dapsone, acedapsone) prevented the multiplication of M. leprae in the mouse foot pad. This provided the rationale for delivering dapsone in an intermittent and fully supervisable fashion for treatment and prophylaxis.
In 1968, Shepard and his colleagues 24 showed that the clinical efficacy could be most sensitively estimated from the loss of viability of M. leprae revealed by inoculation into the mouse foot pad. Not only was viability lost much more rapidly than morphological integrity, but a loss of viability of up to 99% could be monitored in this way, as compared with a loss of about 90% using the MI procedure. Where necessary, biopsy specimens on wet ice can be flown to laboratories thousands of miles away without significant deleterious effects on bacterial viability.25 (For a review of the application of the mouse foot-pad technique to controlled clinical trials in multibacillary leprosy see Levy.26)
The 1970s: Rifampin; dapsone and rifampin resistance; persisters. The activity of rifampin against M. leprae was first demonstrated by Rees and his colleagues in 1970 27 in the mouse foot pad. Their results encouraged them to launch a pilot trial in six patients in whom the MI fell considerably more rapidly than with dapsone treatment. The rapidity of rifampin's bactericidal activity was confirmed in one of the patients by determining the speed with which leprosy bacilli recovered from biopsies lost their infectivity for normal mice. In the following year, Shepard and his colleagues 28 demonstrated the marked bactericidal activity of rifampin when fed to mice for 2 days at a dietary concentration of 0.03% to give blood levels which could readily be attained in man. When discussing their findings, they prophetically suggested the possibility of preventing the emergence of drug-resistant strains of M. leprae by using a combination of fully supervised, large oral doses of rifampin and 225 mg injections of acedapsone given once every 75 days.
The spectacular bactericidal activity of rifampin was confirmed in the following year,29 when Shepard and his colleagues demonstrated the apparent sterilizing activity of one or two doses of 25 mg/kg or 40 mg/kg of rifampin against M. leprae infections in the mouse foot pad, and the complete loss of infectivity of inocula from the first five lepromatous patients after 7 days of daily treatment with 600 mg of rifampin. Further studies in two groups of 14 patients confirmed these remarkable findings; infectivity to mice was lost in every case within 3-7 days of starting daily treatment with 600 mg of rifampin (the time of the first biopsy) or after a single 1500 mg dose of the drug.30
However, in the same year, Waters and his colleagues described a very disquieting phenomenon,31 namely, the isolation of persisting, viable, drug-susceptible bacilli from 7 of 12 lepromatous patients who appeared to have been successfully treated for 10 years with dapsone monotherapy. They therefore concluded that the achievement of smear negativity (BI = 0) was no indication that patients had been cured, and that the practice of giving dapsone for life to multibacillary patients should be continued.
Since the original demonstration of the activity of dapsone in preventing the multiplication of M. leprae in the mouse foot pad by Shepard and Chang in 1962,13 workers had been steadily "titrating down" dapsone's dosage to determine its minimal effective dose and, hence, from concomitant blood-level estimations, its minimal inhibitory concentration (MIC) against M. leprae.23, 32-36 Its MIC against M. leprae was finally shown in both the mouse and rat to be about 2-3 ng/ml.37-38
In 1975, only just over a decade after the discovery of the first cases of dapsone resistance from Sungei Buloh in Malaysia, Pearson and his colleagues 39 reported that 100 cases with proven dapsone-resistant leprosy had been identified at the center. Dapsone resistance occurred, as an average, about 16 years after starting treatment (range 5-24 years), and was favored by initial treatment with low dapsone dosages. The majority of dapsone-resistant strains of M. leprae showed high-grade resistance, being capable of multiplying in mice fed 0.01% dapsone in the diet. Dapsone resistance was only seen among lepromatous patients, and about two thirds of the patients had initially become smear negative prior to relapsing with dapsone-resistant leprosy. Often patients had characteristic new active, asymmetric lesions together with old healed lesions in other parts of the body.
In the following year, there were two other disturbing publications: Jacobson and Hastings 40 reported the first case of proven rifampin-resistant leprosy in a patient from Carville in the U.S.A. who had initially relapsed with dapsone-resistant leprosy after prolonged dapsone monotherapy and had then been treated with rifampin monotherapy. Rees, et al.41 and Waters 42 described the finding of drug-sensitive persisters after up to 5 years of rifampin-containing treatment. The fact that rifampin, with its spectacular bactericidal activity, was no more capable of eliminating persisters than dapsone prompted Rees and his colleagues to conclude their paper by asking whether patients with lepromatous leprosy could ever be cured by chemotherapy alone.
In 1977, Pearson, et al.43 found the first cases of primary dapsone resistance among previously untreated lepromatous patients in Ethiopia. Within another 2 years,44 the spread of dapsone resistance in Ethiopia had been so rapid, with an incidence of acquired (secondary) resistance of about 3% per annum and a prevalence of primary resistance of about 50%, that it seemed likely to jeopardize the possibility of controlling leprosy with dapsone alone.
In 1978, Colston and his colleagues described their proportional bactericidal test method for establishing in the mouse foot pad whether or not compounds displayed bactericidal activity against M. leprae.45 This was a major advance since, because of the very limited ability of lepromatous patients to kill resident leprosy bacilli, bactericidal drugs may be essential for successful chemotherapy.
The 1980s: Multiple drug therapy. In 1981, in response to the increasing evidence of the failure of dapsone monotherapy indicated by the increasing incidence rates of primary and secondary dapsone resistance, the World Health Organization convened a special meeting to consider what the most appropriate response might be. The outcome of the meeting was the recommendation that multibacillary patients should be treated to smear negativity with the triple combination of daily self-administered dapsone and clofazimine supplemented by monthly supervised doses of rifampin and clofazimine.1 It was also recommended that paucibacillary patients should be treated for 6 months with daily dapsone plus monthly rifampin.
The possibility that the failure of rifampin to eliminate persisters did not necessarily imply that lepromatous patients must be treated for life was first suggested by the so-called "Malta trial." This study had been initiated by Freerksen and his colleagues many years earlier,46 when some 80 lepromatous patients previously treated for many years with dapsone monotherapy were prescribed daily rifampin plus dapsone plus prothionamide plus isoniazid for about 2 years. Since isoniazid is thought to be without significant antileprosy activity, it was unlikely that such a regimen would be able to eliminate persisting M. leprae. Nevertheless no relapses were encountered during a period of over 10 years after stopping treatment.47-49 Further encouraging evidence that the treatment of lepromatous patients might be terminated without risking unacceptable relapse rates came in 1986, when Waters, et al.50 described a study from Sungei Buloh in Malaysia in which the treatment of over 300 patients had been terminated after some 20 years of supervised dapsone therapy. Over the following 8 years the relapse rate was only about 1% per annum.
Even more encouraging were the first results from the two THELEP field trials being carried out in South India,51,52 in which not a single relapse had been encountered among over 2000 multibacillary patients during the first 4 years after stopping treatment with the WHO Study Group regimen. When taken with the most recent report from the THELEP Clinical Trials Subcommittee,53 which demonstrated that combinations of rifampin plus dapsone and either clofazimine or prothionamide were unable to eradicate persisters, it could be concluded that the threat posed by persisters may be much less than had been previously supposed.
Antibacterial activity, determination and pharmacology of the most important antileprosy drugs
Dapsone (DDS). The results obtained using both the kinetic and proportional bactericidal test methods showed that dietary concentrations of about 0.01% dapsone, which give blood and tissue drug levels similar to those encountered when treating patients with 50-100 mg of the drug each day, result in pronounced bactericidal activity in experimental infections in both the mouse and rat. By contrast, dietary concentrations of 0.0001% or less are purely bacteriostatci. 17,22,45,54-59 similar activities are found in man. Thus the rates of elimination of viable M. leprae when patients are treated with injections of diacetyldapsone (acedapsone) releasing about 2.4 mg dapsone into the circulation each day 36 are considerably less than when standard daily doses of 50-100 mg dapsone are given.60-62
Dapsone plasma concentrations can readily be determined by specific and sensitive fluorimetric methods63,64 or high pressure liquid chromatography with ultraviolet absorption or fluorescence detection.65,66 The most sensitive procedures are capable of measuring dapsone concentrations of down to about 0.1 ng/ml.67,68
The pharmacokinetics of dapsone have recently been reviewed.69 Dapsone is rapidly and completely absorbed in man.70 The only metabolite demonstrated in the plasma is monoacetyldapsone, and the acetylation of dapsone occurs polymorphically in a fashion similar to that shown by sulfamethazine and isoniazid.71 Slow and rapid acetylators can be readily distinguished according to the ratios of acetylated to free drug in the plasma. However, it is not possible to establish whether monoacetyldapsone has intrinsic antileprosy activity because it is rapidly deacetylated in the mouse.7 Dapsone is eliminated relatively slowly in man, with a half-life averaging a little over 24 hours. Although dapsone half-lives in patients and healthy volunteers may vary from about 15 to 50 hours, they are not related to their acetylator phenotype because monoacetyldapsone is reconverted to dapsone by deacetylation.63,67,72-79
During daily treatment with 100 mg dapsone, plasma and tissue concentrations probably range from about 1 to 2 µg/ml, some 500-fold in excess of its MIC against M. leprae. Because resistance to dapsone is a step-wise phenomenon, it is understandable that treatment with dapsone monotherapy was successful for so long and that full-grade dapsone resistance only emerged after many years of such therapy.80 It is also readily understandable that the emergence of dapsone resistance is unrelated to the patients' acetylator phenotypes or dapsone half-lives.22,76,81,82 The rate of elimination of dapsone is increased by daily rifampin treatments,76 probably by induction of N-hydroxylation.83,84 However, since this is of no therapeutic consequence, it provides no grounds for modifying the recommended dapsone dosage (100 mg per day). The excellent acceptability of dapsone is one of its most important attributes. Laboratory and epidemiological evidence supports the conclusion that neither dapsone nor its human metabolites are carcinogenic,84,85 and that higher doses of dapsone actually aid rather than aggravate, as had been formerly supposed, the control of reactions in patients with borderline disease.86 The mechanism of action of dapsone against M. leprae has been established by the elegant studies of Seydel and his colleagues. Dapsone displayed a very high affinity for the pteroate synthetase of M. leprae,87 but no analog was formed. Since the enzyme from a dapsoneresistant strain was fully susceptible to inhibition by dapsone, it is possible that resistance might be occurring through greatly augmented production of the synthetase.88,89
Rifampin. The remarkable bactericidal action of rifampin against M. leprae described above has been confirmed by other workers in the mouse,45,90-93 rat,94 and in man.95 Levy, et al.96 investigated the bactericidal effects of single doses of rifampin in lepromatous patients by mouse foot-pad inoculation and found only suggestive evidence that doses of 1200-1500 mg might be slightly more bactericidal than doses of 600-900 mg (p = 0.05).
Absorption of rifampin is generally excellent, although some studies have suggested that it may be impaired if rifampin is not taken on an empty stomach.97 Its rate of elimination is dependent on dose size, and repeated daily dosage leads to induction of metabolism and more rapid clearance from the body. Single oral doses of 600 mg are eliminated with a half-life of about 3-4 hours. A number of high-pressure liquid chromatographic methods have been described for its determination,65 and serum concentrations can also be readily estimated using a microbiological plate diffusion assay with Staphylococcus aureus.98
When given at a daily dose of 600 mg, as in the treatment of tuberculosis, rifampin is excellently tolerated.99,100 Once-weekly treatment with larger doses (900-1200 mg), however, resulted in immunologically based toxicity, especially the so-called "flu" syndrome.101 Fortunately, when monthly doses of 600 or 1200 mg, or pairs of 600 mg doses given on consecutive days every month, were used for the treatment of leprosy, they were excellently tolerated.51,52,102-106
Clofazimine. The following observations suggested that supervised intermittent clofazimine treatment might be clinically effective: clofazimine's very low minimal effective dose (0.0001%) against M. leprae in the mouse,107 its activity when given in the diet for 2 days every month,108 its bacterio pausal or bactericidal activity when tested by the kinetic method,55,109 together with its marked tissue accumulation and slow elimination from both mouse and man.32,110-117 It was also shown to have unequivocal bactericidal activity in the mouse when tested by the proportional bactericidal test method.43,118
The efficacy of daily treatment with 300 mg clofazimine, demonstrated by studies of the fall of the MI, 18,119 was confirmed by a mouse foot-pad inoculation study in which patients were treated with daily doses of 100-200 mg of the drug.120 Since the rate of loss of viability of M. leprae was faster than when patients were treated with acedapsone 68 (a fully effective bacteriostatic drug), it is apparent that clofazimine also displays bactericidal activity in man. Treatment with 600 mg doses of clofazimine given on 2 consecutive days once very month was shown to be bacteriologically efficacious, although the rate of killing of M. leprae was slower than when the same total dose was given thrice or once weekly.121
The activity of daily treatment with 300 mg of the drug in controlling ENL was confirmed by Hastings and Trautman 122 and Helmy, et al.123 Light-skinned patients sometimes find the skin pigmentation caused by clofazimine unacceptable, especially if large doses are used.119 However, lower doses (50-100 mg per day) are generally well tolerated 124 and are also accompanied by a significant reduction in the frequency of ENL.
Sensitive and specific high-pressure liquid and thin-layer chromatographic methods have been described for its determination in plasma by Lanyi and Dubois 116 and Peters, et al.,125 while its metabolism has been studied by Feng, et al.115 The essential insolubility of clofazimine in water and the recovery of a large portion of the drug in the feces, apparently in an unmetabolized form, strongly suggest that its absorption from the gut is incomplete,111,112 and it is highly likely that the proportion of the dose absorbed decreases as the individual dose size is increased.
Other extensively investigated antimycobacterial drugs. The activity of the two thioamides, ethionamide and prothionam ide against M. leprae in the mouse foot pad and their pharmacokinetics in the mouse and in man are extremely similar. Dietary doses of 0.01% inhibit multiplication in the mouse foot pad,107,126 their MIC is about 0.05 µg/ml and both possess marked bactericidal activity when tested by the kinetic and proportional bactericidal test methods. 45,54,55,59,118,126-128 Their bactericidal activity was abolished when they were given on a once-weekly basis,129 suggesting that their clinical efficacy could be threatened by poor patient compliance. The antileprosy activity of their sulfoxide metabolites is probably similar to that of the parent thioamides.130 Both thioamides are excellently absorbed in man, and they are then rapidly eliminated from the body with a half-life of about 2 hours.131 Prior to the THELEP trial currently being carried out in Cebu in The Philippines, to compare the bactericidal activities of ethionamide and prothionamide, there is only a single report of their therapeutic activity in man having been assessed by mouse foot-pad inoculation.62 Three patients had been treated with 250 mg ethionamide three times a day, and there was a rapid loss in bacterial viability, suggesting that in man, as in the mouse, the thioamides are probably more rapidly bactericidal than dapsone. The two thioamides can be determined in the plasma by high pressure liquid chromatographic methods.132, 133
Both thioamides were used for the treatment of pulmonary tuberculosis prior to the introduction of rifampin. Gastrointestinal side effects were common, although prothionamide appeared to be better tolerated 134 However, the daily dosages used (500-1000 mg) were higher than those (250-375 mg) recommended for leprosy treatment.1 Little is known about the acceptability of the thioamides when given at these lower dosages, although recent reports concerning their hepatotoxicity when combined with rifampin 135-138 and gastrointestinal side effects 139 give cause for concern.
Thiacetazone is a weak bacteriostatic drug whose antileprosy activity in the mouse foot pad is virtually abolished when it is given once a week.129,140 Although thiacetazone has a relatively long half-life in man, it was estimated that plasma and tissue concentrations would decline to subinhibitory levels within about 3 days after discontinuing treatment, making it an unsuitable drug for self-administration.141-143 Isoniazid has been used fairly extensively in some parts of the world for the treatment of leprosy in a commercially available combined formulation with dapsone and prothionamide.46 Although its routine use should be discouraged, since isoniazid is without significant antileprosy activity,22,55 such a formulation could be of value when given in a more restricted way for monitoring prothionamide compliance.139
- Gordon A. Ellard, Ph.D.
National Institute for Medical Research
The Ridgeway, Mill Hill
London NW7 1AA, England
1. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
2. Lopez-Bravo, L. L'évolution de l'éndemie lépreuse dans le monde. Acta Leprol. 101(1986)129-139.
3. World Health Organization. A Guide to Leprosy Control. 2nd ed. Geneva: World Health Organization, 1988.
4. Sutherland, I. The epidemiology of tuberculosisis prevention better than cure? Bull. Int. Union Tuberc. 56(1981)127-134.
5. Sutherland, I. Research into the control of tuberculosis and leprosy in the community. Br. Med. Bull. 44(1988)665-678.
6. Dominguez, V. M., Garbajosa, P. G., Gyi, M. M., Tamondong, C. T., Sundaresan, T., Bechelli, L. M., Lwin, K., Sansarricq, H., Walter, J. and Noussitou, F. M. Epidemiological information on leprosy in the Singu area of Upper Burma. Bull. WHO 58(1980)81-89.
7. Ponnighaus, J. M., Fine, P. E. M., Maine, N., Bliss, L., Kalambo, M. and Ponnighaus, I. The Lepra Evaluation Project (LEP), an epidemiological study of leprosy in northern Malawi. II: Prevalence rates. Lepr. Rev. 59(1988)97-112.
8. Faget, G. H., Pogge, R. C, Johansen, F. A., Dinan, J. F., Prejean, B. M. and Eccles, C. G. The Promin treatment of leprosy; a progress report. Public Health Rep. 58(1943)1729-1741.
9. Lowe, J. and Smith, M. The chemotherapy of leprosy in Nigeria. Int. J. Lepr. 17(1949)181-195.
10. Ridley, D. S. Bacterial indices. In: Leprosy in Theory and Practice. 2nd ed. Cochrane, R. G. and Davey, T. F., eds. Bristol: John Wright, 1964, p. 620.
11. Waters, M. F. R. and Rees, R. J. W. Changes in the morphology of Mycobacterium leprae in patients under treatment. Int. J. Lepr. 30(1962)266-277.
12. Shepard, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112(1960)445-454.
13. Shepard, C. C. and Chang, Y. T. Effect of several anti-leprosy drugs on multiplication of human leprosy bacilli in footpads of mice. Proc. Soc. Exp. Biol. Med. 109(1962)636-638.
14. Pettit, J. H. and Rees, R. J. W. Sulphone resistance in leprosy; an experimental and clinical study. Lancet 2(1964)673-674.
15. Shepard, C. C. and McRae, D. H. Mycobacterium leprae in mice: minimal infectious dose, relationship between staining quality and infectivity, and effect of cortisone. J. Bacteriol. 89(1965)365-372.
16. Levy, L. Studies of the mouse footpad technique for cultivation of Mycobacterium leprae. 3. Doubling time during logarithmic multiplication. Lepr. Rev. 47(1976)103-106.
17. Levy, L. and Peters, J. H. Some characteristics of the action of dapsone on multiplication of Mycobacterium leprae in the mouse. Lepr. Rev. 48(1977)237-245.
18. Pettit, J. H. and Rees, R. J. W. Studies on sulfonc resistance in leprosy. 2. Treatment with a riminophenazine derivative (B.663). Int. J. Lepr. 34(1966)391-397.
19. Browne, S. G. B 663; possible anti-inflammatory action in lepromatous leprosy. Lepr. Rev. 36(1965)9-11.
20. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
21. Rees, R. J. W. Enhanced susceptibility of thymectomized and irradiated mice to infection with Mycobacterium leprae. Nature 211(1966)657-658.
22. Shepard, C. C. A kinetic method for the study of activity of drugs against Mycobacterium leprae in mice. Int. J. Lepr. 35(1967)429-435.
23. Shepard, C. C. Activity of repository sulfones against Mycobacterium leprae in mice. Proc. Soc. Exp. Biol. Med. 124(1967)430-433.
24. Shepard, C. C, Levy, L. and Fasal, P. The death of Mycobacterium leprae during treatment with 4,4'diaminodiphenylsulfone (DDS). Am. J. Trop. Med. Hyg. 17(1968)769-775.
25. Baras, M. and Levy, L. EfTect of shipment on skin-biopsy specimens to a distant laboratory on viability of Mycobacterium leprae. Int. J. Lepr. 56(1988)115-118.
26. Levy, L. Application of the mouse foot-pad technique in immunologically normal mice in support of clinical drug trials, and a review of earlier clinical drug trials in lepromatous leprosy. Int. J. Lepr. 55 Suppl.(1987)823-829.
27. Rees, R. J. W., Pearson, J. M. H. and Waters, M. F. R. Experimental and clinical studies on rifampicin in the treatment of leprosy. Br. Med. J. 1(1970)89-92.
28. Shepard, C. C, Walker, L. L., Van Landingham, R. M. and Redus, M. Kinetic testing of drugs against Mycobacterium leprae in mice; activity of cephaloridine, rifampin, streptovaricin, and viomycin. Am. J. Trop. Med. Hyg. 20(1971)616-620.
29. Shepard, C. C, Levy, L. and Fasal, P. Rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21(1972)446-449.
30. Shepard, C. C, Levy, L. and Fasal, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1124.
31. Waters, M. F. R., Rees, R. J. W., McDougall, A. C. and Weddell, A. G. M. Ten years of dapsone in lepromatous leprosy: clinical, bacteriological and histological assessment and the finding of viable leprosy bacilli. Lepr. Rev. 45(1974)288-298.
32. Shepard, C. C. and Chang, Y. T. Activity of antituberculosis drugs against Mycobacterium leprae; studies with experimental infection in mouse footpads. Int. J. Lepr. 32(1964)260-271.
33. Shepard, C. C, McRae, D. H. and Habas, J. A. Sensitivity of Mycobacterium leprae to low levels of 4,4'-diaminodiphenyl sulfone. Proc. Soc. Exp. Biol. Med. 122(1966)893-896.
34. Shepard, C. C, Levy, L. and Fasal, P. The sensitivity to dapsone (DDS) of Mycobacterium leprae from patients with and without previous treatment. Am. J. Trop. Med. Hyg. 18(1969)258-263.
35. Ellard, G. A., Gammon, P. T., Rees, R. J. W. and Waters, M. F. R. Studies on the determination of the minimal inhibitory concentration of 4,4'-diamino-diphenyl-sulphone (Dapsone, DDS) against Mycobacterium leprae. Lepr. Rev. 42(1971)101-117.
36. Ozawa, T., Shepard, C. C. and Karat, A. B. A. Application of spectrophotofluorometric procedures to some problems in Mycobacterium leprae infections in mice and man treated with dapsone (DDS), diacetyl-DDS (DADDS) and di-formyl-DDS (DFD). Am. J. Trop. Med. Hyg. 20(1971)274-281.
37. Peters, J. H., Gordon, G. R., Murray, J. F., Fieldsteel, A. H. and Levy, L. Minimal inhibitory concentrations of dapsone for Mycobacterium leprae in rats. Antimicrob. Agents Chemother. 8(1975)551-557.
38. Levy, L. and Peters, J. H. Susceptibility of Mycobacterium leprae to dapsone as a determinant of patient response to acedapsone. Antimicrob. Agents Chemother. 9(1976)102-112.
39. Pearson, J. M. H., Rees, R. J. W. and Waters, M. F. R. Sulphone resistance in leprosy; a review of one hundred proven clinical cases. Lancet 2(1975)69-72.
40. Jacobson, R. R. and Hastings, R. C. Rifampicinresistant leprosy. Lancet 2(1976)1304-1305.
41. Rees, R. J. W., Waters, M. F. R., Pearson, J. M. H., Helmy, H. S. and Laing, A. B. G. Long-term treatment of dapsone-resistant leprosy with rifampicin: clinical and bacteriological studies. Int. J. Lepr. 44(1976)159-169.
42. Waters, M. F. R. The diagnosis and management of dapsone-resistant leprosy. Lepr. Rev. 48(1977)95-105.
43. Pearson, J. M. H., Haile, G. S. and Rees, R. J. W. Primary dapsone-resistant leprosy. Lepr. Rev. 48(1977)129-132.
44. Pearson, J. M. H., Haile, G. S., Barnetson, R. St. C. and Rees, R. J. W. Dapsone-resistant leprosy in Ethiopia. Lepr. Rev. 50(1979)183-199.
45. Colston, M. J., Hilson, G. R. F. and Banerjee, D. K. The "proportional bactericidal test;" a method for assessing bactericidal activity of drugs against Mycobacterium leprae in mice. Lepr. Rev. 49(1978)7-15.
46. Freerksen, E., Rosenfeld, M., Bonnici, E., Depasquale, G. and Kruger-Thiemer, M. Combined therapy in leprosy, background and findings. Chemotherapy 24(1978)187-201.
47. Jopling, W. H., Ridley, M., Bonnici, E. and Depasquale, G. A follow-up investigation of the Malta Project. Lepr. Rev. 55(1984)247-253.
48. Jopling, W. H. A report on two follow-up investigations of the Malta-Project. Lepr. Rev. 57 Suppl.(1986)47-52.
49. Leiker, D. L. First assessment of the Malta Leprosy Eradication Project. Lepr. Rev. 57 Suppl.(1986)42-46.
50 Waters, M. F. R., Rees, R. J. W., Laing, A. B. G., Fah, K. K., Meade, T. W., Parikshak, N. and North, W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulphone therapy. Lepr. Rev. 57(1986)101-109.
51. UNDP/World BankAVHO Special Programme for Research and Training in Tropical Diseases. Tropica! disease research: a global partnership. Eighth programme report. Maurice, J. and Pearce, A. M., eds. Geneva: World Health Organization, 1987.
52. UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Tropical diseases: progress in international research, 1987-1988. Ninth programme report. Geneva: World Health Organization, 1989, pp. 97-99.
53. HELEP Subcommittee on Clinical Trials of the Chemotherapy of Leprosy Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
54. Shepard, C. C. Further experience with the kinetic method for the study of drugs against Mycobacterium leprae in mice; activities of DDS, DFD, ethionamide, capreomycin, and PAM 1392. Int. J. Lepr. 37(1969)389-397.
55. Shepard, C. C. Combinations of drugs against Mycobacterium leprae studied in mice. Int. J. Lepr. 40(1972)33-39.
56. Levy, L., Ng, H., Evans, M. J. and Krahenbuhl, J. L. Susceptibility of thymectomized and irradiated mice to challenge with several organisms and the effect of dapsone on infection with Mycobacterium leprae. Infect. Immun. 11(1975)1122-1132.
57. Fieldsteel, A. H. and Levy, L. Dapsone chemotherapy of Mycobacterium leprae infections of the neonatally thymectomized Lewis rat. Am. J. Trop. Med. Hyg. 25(1976)854-859.
58. Levy, L. Bactericidal action of dapsone against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 9(1976)614-617.
59. Pattyn, S. R., Portaels, F., Van Loo, G. and Van den Breen, L. Activity of the combination of isoniazid, protionamide and dapsone against Mycobacterium leprae and some other mycobacteria. Arzneimittelforschung 31(1981)2155-2157.
60. Shepard, C. C, Levy, L. and Fasal, P. The death rate of Mycobacterium leprae during treatment of lepromatous leprosy with acedapsone (DADDS). Am. J. Trop. Med. Hyg. 21(1972)440-445.
61. Russell, D. A., Shepard, C. C, McRae, D. H., Scott, G. C. and Vincin, D. R. Acedapsone (DADDS) treatment of leprosy patients in the Karimui of Papua New Guinea; status at six years. Am. J. Trop. Med. Hyg. 24(1975)485-495.
62. Shepard, C. C. A brief review of experiences with short-term clinical trials monitored by mouse foot-pad inoculation. Lepr. Rev. 52(1981)299-308.
63. Glazko, A. J., Dill, W. A., Montalbo, R. G. and Holmes, E. L. A new analytical procedure for dapsone; application to blood-level and urinary-excretion studies in normal men. Am. J. Trop. Med. Hyg. 17(1968)465-473.
64. Peters, J. H., Gordon, G. R. and Colwell, W. T. The fluorometric measurement of 4,4'-diaminodiphenyl sulfone and its acetylated derivatives in plasma and urine. J. Lab. Clin. Med. 76(1970)338-348.
65. Jenner, P. J. The determination of antimycobacterial drugs in body fluids by high-performance liquid chromatography. In: Progress in Medical Microbiology. Reeves, D. and Ullman, U., eds. Stuttgart: Fischer, 1984, Vol. 2, pp. 153-164.
66. Pieters, F. A. J. M., Vinckcn, B. J. and Zuidema, J. Dapsone and monoacetyldapsone determined in serum and saliva by a sensitive high-performance liquid chromatographic method with a single extraction step. J. Chromatogr. 422(1987)322-327.
67. Peters, J. H., Murray, J. F., Gordon, G. R. and Gelber, R. H. Dapsone in saliva and plasma of man. Pharmacology 22(1981)162-171.
68. Murray, J. F., Gordon, G. R., Gulledge, C. C. and Peters, J. H. Chromatographic-fluorometric analysis of antileprotic sulfones. J. Chromatogr. 107(1975)67-72.
69. Zuidema, J., Hilbers-Modderman, E. S. M. and Merkus, F. W. H. M. Clinical pharmacokinetics of dapsone. Clin. Pharmacokin. 11(1986)299-315.
70. Pieters, F. A. J. M. and Zuidema, J. The absolute oral bioavailability of dapsone in dogs and humans. Int. J. Clin. Pharmacol. Ther. Toxicol. 25(1987)396-400.
71. Levy, L., Biggs, J. T., Gordon, G. R. and Peters, J. H. Disposition of the antileprosy drug dapsone in the mouse. Proc. Soc. Exp. Biol. Med. 140(1972)937-943.
72. Gelber, R., Peters, J. H., Gordon, G. R., Glazko, A. J. and Levy, L. The polymorphic acetylation of dapsone in man. Clin. Pharmacol. Ther. 12(1971)225-238.
73. Peters, J. H., Gordon, G. R., Ghoul, D. C, Tolentino, J. G., Walsh, G. P. and Levy, L. The disposition of the antileprotic drug dapsone (DDS) in Philippine subjects. Am. J. Trop. Med. Hyg. 21(1972)450-457.
74. Peters, J. H., Gordon, G. R. and Karat, A. B. A. Polymorphic acetylation of the antibacterials, sulfamethazine and dapsone, in South Indian subjects. Am. J. Trop. Med. Hyg. 24(1975)641-648.
75. Peters, J. H., Gordon, G. R., Murray, J. F. and Meyers, W. M. Metabolic disposition of dapsone in African leprosy patients. Lepr. Rev. 50(1977)7-19.
76. Gelber, R. H. and Rees, R. J. W. Dapsone metabolism in patients with dapsone-resistant leprosy. Am. J. Trop. Med. Hyg. 24(1975)963-967.
77. Lammintausta, K., Kangas, L. and Lammintausta, R. The pharmocokinetics of dapsone and acetylated dapsone in serum and saliva. Int. J. Clin. Pharmacol. Biopharm. 17(1979)159-163.
78. Ahmad, R. A. and Rogers, H. J. Pharmacokinetics and protein binding interactions of dapsone and pyrimethamine. Br. J. Clin. Pharmacol. 10(1980)519-524.
79. Pieters, F. A. J. M. and Zuidema, J. The pharmacokinetics of dapsone after oral administration to healthy volunteers. Br. J. Clin. Pharmacol. 22(1986)491-494.
80. Ellard, G. A. Rationale of the multidrug regimens recommended by a World Health Organization study group of chemotherapy of leprosy for control programs. Int. J. Lepr. 52(1984)395-401.
81. Ellard, G. A., Gammon, P. T., Helmy, H. S. and Rees, R. J. W. Dapsone acetylation and the treatment of leprosy. Nature 239(1972)159-160.
82. Peters, J. H., Shepard, C. C, Gordon, G. R., Rojas, A. V. and Elizondo, D. S. The incidence of DDS resistance in lepromatous patients in Costa Rica: their metabolic disposition of DDS. Int. J. Lepr. 44(1976)143-151.
83. Israili, Z. H., Cucinell, S. A., Vaught, J., Davis, E., Lesser, J. M. and Dayton, P. G. Studies of the metabolism of dapsone in man and experimental animals: formation of N-hydroxy metabolites. J. Pharmacol. Exp. Ther. 187(1973)138-151.
84. Gordon, G. R., Murray, J. F., Peters, J. H. Gelber, R. H. and Jacobson, R. R. Studies on the urinary metabolites of dapsone in man. Int. J. Lepr. 47(1979)681-682.
85. Brinton, L. A., Hoover, R., Jacobson, R. R. and Fraumeni, J. F. Cancer mortality among patients with Hansen's disease. J. Natl. Cancer Inst. 72 (1984) 109-114.
86. Barnetson, R. St. C, Pearson, J. M. H. and Recs, R. J. W. Evidence for prevention of borderline leprosy reactions by dapsone. Lancet 2(1976)1171-1172.
87. Kulkarni, V. M. and Seydel, J. K. Inhibitory activity and mode of action of diaminodiphenylsulfone in cell-free folate synthesizing systems prepared from Mycobacterium lufil and Mycobacterium leprae. Chemotherapy 29(1983)58-67.
88. Coats, E. A., Cordes, H.-P., Kulkarni, V. M., Richter, M., Schaper, K.-J., Wiese, M. and Seydel, J. K. Multiple regression and principal component analysis of antibacterial activities of sulfones and sulfonamides in whole cell and cell-free systems of various DDS sensitive and resistant bacterial strains. Quant. Struct. Act. Relations. 4(1985)99-109.
89. Seydel, J. K., Schaper, K. J., Wiese, M., Rosenfeld, M., Hachtel, G., Haller, R., Kansy, M. and Dhople, M. The development of new sulphones, benzylpyrimidines and hydrazones as inhibitors of mycobacterial (including M. leprae) folate synthesis and nucleotide diphosphate reductase, respectively. Health Coop. Papers 7(1988)239-276.
90. Holmes, I. B. and Hilson, G. R. F. The effect of rifampicin and dapsone on experimental Mycobacterium leprae infections; minimum inhibitory concentrations and bactericidal action. J. Med. Microbiol. 5(1972)251-261.
91. Holmes, I. B. and Hilson, G. R. F. The rate of bactericidal action of rifampin on Mycobacterium leprae in the mouse footpad. Proc. Soc. Exp. Biol. Med. 145(1974)1395-1400.
92. Holmes, I. B., Banerjcc, D. K. and Hilson, G. R. F. Effect of rifampicin, clofazimine, and B 1912 on the viability of Mycobacterium leprae in established mouse foot-pad infection. Proc. Soc. Exp. Biol. Med. 151(1976)637-641.
93. Pattyn, S. R. A comparison of the bactericidal activity of a series of rifampicins against Mycobacterium leprae. Arzneimittclforschung 32(1982)15-17.
94. Fieldsteel, A. H. and Levy, L. Combined rifampin and dapsone chemotherapy of Mycobacterium leprae infection of the neonatally thymectomized Lewis rat. Int. J. Lepr. 48(1980)267-276.
95. Collaborative effort of the U.S. Leprosy Panel and the Leonard Wood Memorial. Rifampin therapy in lepromatous leprosy. Am. J. Trop. Med. Hyg. 24(1975)475-484.
96. Levy, L., Shepard, C. C. and Fasal, P. The bactericidal effect of rifampicin on M. leprae in man: (a) Single doses of 600, 900 and 1200 mg; (b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
97. Kenney, M. T. and Strates, B. Metabolism and pharmacokinetics of the antibiotic rifampin. Drug Metabol. Rev. 12(1981)159-218.
98. Dickinson, J. M., Abcr, V. R., Allen, B. W., Ellard, G. A. and Mitchison, D. A. Assay of rifampicin in serum. J. Clin. Pathol. 27(1974)457-462.
99. Girling, D. J. Adverse reactions to rifampicin in antituberculosis regimens. J. Antimicrob. Chemother. 3(1977)115-132.
100. Grosset, J. and Leventis, S. Adverse effects of rifampin. Rev. Infect. Dis. 5 Suppl.(1983)S440-S446.
101. Aquinas, M., Allan, W. G. L., Horsfall, P. A. L., Jenkins, P. K., Hung-Yan, W., Girling, D., Tall, R. and Fox, W. Adverse reactions to daily and intermittent rifampicin regimens for pulmonary tuberculosis in Hong Kong. Br. Med. J. 1(1972)765-771.
102. Rees, R. J. W. Rifampicin: the investigation of a bactericidal antileprosy dryg. Lepr. Rev. 46 Suppl.(1975)121-124.
103.Yawalkar, S. J., McDougall, A. C, Languillon, J., Ghosh, S., Hajra, S. K., Opromolla, D. V. A. and Tonello, C. J. S. Once-monthly rifampicin plus daily dapsone in initial treatment of lepromatous leprosy. Lancet 1(1982)1199--1202.
104. Rose, P. Short-course multi-drug therapy for paucibacillary patients in Guyana: preliminary communication. Lepr. Rev. 55(1984)143-147.
105. Boerrigter, G., Ponnighaus, J. M. and Fine, P. E. M. Preliminary appraisal of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
106. Levy, L. Chairman's report of chemotherapy precongress workshop. 13th International Leprosy Congress, The Hague, The Netherlands. Lepr. Rev. 59(1988)281-283.
107. Shepard, C. C. Minimal effective dosages in mice of clofazimine (B663) and of ethionamide against Mycobacterium leprae. Proc. Soc. Exp. Biol. Med. 132(1969)120-124.
108. Shepard, C. C, Walker, L. L., Van Landingham, R. M. and Redus, M. A. Discontinuous administration of clofazimine (B.663) in Mycobacterium leprae infections. Proc. Soc. Exp. Biol. Med. 137(1971)725-727.
109. Shepard, C. C. Combinations involving dapsone, rifampin, clofazimine, and ethionamide in the treatment of M. leprae infections in mice. Int. J. Lepr. 44(1976)135-139.
110. Barry, V. C. Boyle Medal Lecture: Synthetic phenazine derivatives and mycobacterial disease: a twenty year investigation. Sci. Proc. R. Dublin Soc. Ser. A 3(1969)153-170.
111. Banerjee, D. K, Ellard, G. A., Gammon, P. T. and Waters, M. F. R. Some observations on the pharmacology of clofazimine. Am. J. Trop. Med. Hyg. 23(1974)1110-1115.
112. Levy, L. Pharmacologic studies of clofazimine. Am. J. Trop. Med. Hyg. 23(1974)1097-1109.
113. Mansfield, R. E. Tissue concentrations of clofazimine (B663) in man. Am. J. Trop. Med. Hyg. 23(1974)1116-1119.
114. Balakrishnan, S., Desikan, K. V. and Ramu, G. Quantitative estimation of clofazimine in tissues. Lepr. India 48 Suppl.(1976)732-738.
115. Feng, P. C. C, Fenselau, C. C. and Jacobson, R. R. Metabolism of clofazimine in leprosy patients. Drug Metab. Dispos. 9(1981)521-524.
116. Lanyi, Z. and Dubois, J. P. Determination of clofazimine in human plasma by thin-layer chromatography. J. Chromatogr. 232(1982)219-232.
117. Schaad-Lanyi, Z., Dieterle, W., Dubois, J.-P., Theobald, W. and Vischer, W. Pharmacokinetics of clofazimine in healthy volunteers. Int. J. Lepr. 55(1987)9-15.
118. Gelber, R. H. The killing of Mycobacterium leprae in mice by various dietary concentrations of clofazimine and ethionamide. Lepr. Rev. 58(1987)407-411.
119. Pettit, J. H., Rees, R. J. W. and Ridley, D. S. Chemotherapeutic trials in leprosy. 3. Pilot trial of a riminophenazine derivative, B.663, in the treatment of lepromatous leprosy. Int. J. Lepr. 35(1967)25-33.
120. Levy, L., Shepard, C. C. and Fasal, P. Clofazimine therapy of lepromatous leprosy caused by dapsone-resistant Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21(1972)315-321.
121. Collaborative effort of the U.S. Leprosy Panel and the Leonard Wood Memorial. Spaced clofazimine therapy of lepromatous leprosy. Am. J. Trop. Med. Hyg. 25(1976)437-444.
122. Hastings, R. C. and Trautman, J. R. B663 in lepromatous leprosy; effect in erythema nodosum leprosum. Lepr. Rev. 39(1968)3-7.
123. Helmy, H. S., Pearson, J. M. H. and Waters, M. F. R. Treatment of moderately severe erythema nodosum lcprosum with clofazimine-a controlled trial. Lepr. Rev. 42(1972)167-177.
124. Hastings, R. C, Jacobson, R. R., and Trautman, J. R. Long-term clinical toxicity studies with clofazimine (B663) in leprosy. Int. J. Lepr. 44(1976)287-293.
125. Peters, J. H., Hamme, K. J. and Gordon, G. R. Determination of clofazimine in plasma by high-performance liquid chromatography. J. Chromatogr. 229(1982)503-508.
126. Colston, M. J., Ellard, G. A. and Gammon, P. T. Drugs for combined therapy: experimental studies on the antileprosy activity of ethionamide and prothionamide, and a general review. Lepr. Rev. 49(1978)115-126.
127. Pattyn, S. R. and Van Loo, G. Combined chemotherapy against Mycobacterium leprae in the mouse. Ann. Soc. Belg. Med. Trop. 60(1980)291-295.
128. Shepard, C. C, Jenner, P. J., Ellard, G. A. and Lancaster, R. D. An experimental study of the antileprosy activity of a series of thioamides in the mouse. Int. J. Lepr. 53(1985)587-594.
129. Colston, M. J., Hilson, G. R. F. and Lancaster, R. D. Intermittent chemotherapy of experimental leprosy in mice. Am. J. Trop. Med. Hyg. 29(1980)103-108.
130. Matsuo, Y., Tatsukawa, H., Murray, J. F. and Peters, J. H. Prothionamide and prothionamide-S-oxide in experimental leprosy. Int. J. Lepr. 49(1981)302-306.
131. Jenner, P. J. and Smith, S. E. Plasma levels of ethionamide and prothionamide in a volunteer following intravenous and oral dosages. Lepr. Rev. 58(1987)31-37.
132. Jenner, P. J. and Ellard, G. A. High-performance liquid chromatographic determination of ethionamide and prothionamide in body fluids. J. Chromatogr. 225(1981)245-251.
133. Jenner, P. J., Ellard, G. A., Grucrt, P. J. K. and Aber, V. R. Comparison of the blood levels and urinary excretion of ethionamide and prothionamide in man. J. Antimicrob. Chemother. 13(1984)267-277.
134. Fox, W., Robinson, D. K., Tall, R., Mitchison, D. A., Kent, P. W. and Macfadycn, D. M. A study of acute tolerance to ethionamide, including a comparison with prothionamide, and of the influence of a vitamin B-complex additive in prophylaxis. Tubercle 50(1969)125-143.
135. Ji, B., Chen, J., Wang, C. and Xia, G. Hepatotoxicity of combined therapy with rifampicin and daily prothionamide for leprosy. Lepr. Rev. 25(1984) 283-289.
136. Pattyn, S. R., Janssens, L., Bourland, J., Saylan, T., Davies, E. M., Grillone, S., Feracci, C, and the Collaborative Study Group of the Treatment of Leprosy. Hepatotoxicity of the combination of rifampinethionamide in the treatment of multibacillary leprosy. Int. J. Lepr. 52(1984)1-6.
137. Cartel, J. L., Millan, J., Guelpa-Lauras, C. C. and Grosset, J. H. Hepatitis in leprosy patients treated with a daily combination of dapsone, rifampin and a thioamide. Int. J. Lepr. 51(1983)461-465.
138. Cartel, J., Naudillon, Y., Artus, J. and Grosset, J. Hepatotoxicity of the daily combination of 5 mg/kg prothionamide + 10 mg/kg rifampin. Int. J. Lepr. 53(1985)15-18.
139. Ellard, G. A., Kiran, K. U. and Stanley, J. N. A. Long-term prothionamide compliance: a study carried out in India using a combined formulation containing prothionamide, dapsone and isoniazid. Lcpr. Rev. 59(1988)163-175.
140. Colston, M. J., Hilson, G. R. F., Ellard, G. A., Gammon, P. T. and Rees, R. J. W. The activity of thiacetazone, thiambutosine, thiocarlide and sulphamethoxypyridazine against Mycobacterium leprae in mice. Lepr. Rev. 49(1978)101-113.
141. Jenner, P. J. High-performance liquid chromatographic determination of thiacetazone in body fluids. J. Chromatogr. 276(1983)463-470.
142. Jenner, P. J., Ellard, G. A. and Swai, O. B. A study of thiacetazone blood levels and urinary excretion in man, using high performance liquid chromatography. Lepr. Rev. 55(1984)121-128.
143. Ji, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
* We are fortunate to have the opportunity of publishing this authoritative review of the chemotherapy of leprosy by Dr. Gordon Ellard. Due to space constraints, it will appear in two parts, the first part in this issue and the second part in the March 1991 issue-RCH.