- Volume 58 , Number 4
- Page: 726–9
New findings on the mode of entry of Mycobacterium leprae in nude mice
To The Editor:
In a study reported by us earlier, nude mice in groups of 10 were exposed to Mycobacterium leprae by subcutaneous injection, and topically on the mucosa of the nose, lungs, mouth, stomach, and broken and unbroken skin. Leprosy infection was transmitted only to those animals exposed topically through the nasal mucosa or to those animals injected with M. leprae subcutaneously (1). These findings were later confirmed by Lancaster (4).
It is well known that in humans M. leprae has a special predilection for the cooler regions of the body with a temperature of 30ºC to 35ºC. M. leprae are routinely grown in the foot pads of mice, and this is thought to be because the temperature of the foot pad is around 30ºC at an ambient temperature of 20ºC-25ºC (7). It was realized that in our earlier experiment M. leprae were introduced into the skin of the flanks of the mice, an area with a temperature higher than that of the feet. It is possible that the infection failed to take because of the higher temperature at the site of infection. Therefore, in our present study it was planned to introduce the organism on the skin of the feet of mice, a site which may be more suited for the growth of M. leprae.
Five female athymic nude (nu/nu) mice approximately 6 weeks old, housed in a laminar flow environment at a temperature of 25ºC-27ºC and humidity of 55%, were used for the experiment. The animals were fed with autoclaved laboratory rodent chow (Tek-Lab (HSD), Madison, Wisconsin, U.S.A.) fortified with vitamins and autoclaved water. Sterilized bedding was used and changed once weekly. Details of the animal husbandry procedures have been described earlier (2).
The animals were anesthetized with 60 mg/kg body weight of sodium pentobarbital intraperitoneally, and the dorsal aspects of both feet were abraded using a sterile corncallus file covering an area of about 0.5 cm2, taking care not to draw blood. Twenty µl containing a total of 3.5 x 108 freshly harvested nude-mouse-derived M. leprae was applied to the abraded areas of the dorsum of both feet as follows: The first 10 µl of the suspension was applied and was allowed to dry using an air draft from a hair dryer. Then the other 10 µl was added and the area was again dried. This was followed by an application of 2% aqueous gelatin on the abraded skin. After recovering from anesthesia, the animals were released into a cage and observed for signs of infection.
One animal was sacrificed at 9 months. Both feet were harvested for M. leprae and were found negative. No histopathological studies were done with the tissues of this animal. Two animals were found dead at 19 and 20 months. Their tissues were decomposed and were unsuitable for study. In the other two animals, swelling of the dorsum of the left foot in one and the right foot in the other gradually developed. The swelling became pronounced and nodular at 22 months (Fig. 1). The animals were obviously lethargic and were sacrificed. A piece from each of the swollen feet of the two animals was taken and was used for bacterial counts. They were autopsied; tissues were removed from various organs, were immediately fixed in 10% buffered Formalin, and were processed for paraffin sections. Five µm sections were cut and stained with hematoxylin and eosin and a modified Fite-Faraco stain for M. leprae (3).
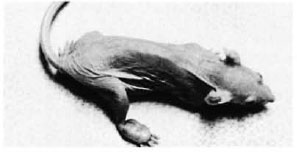
Fig. 1. Nude mouse with a well-formed nodular swelling on the dorsum of the right hind foot.
The nodular swellings measured approximately 0.7 x 0.4 x 0.3 cm in the right foot of animal no. 1 (Fig. 1) and 0.6 x 0.4 x second animal also had small nodules measuring 0.3 cm in diameter in both ears. The animals otherwise had a normal appearance. The bacterial count done from a piece of the nodule removed from the foot of animal no. 1 was 2.8 x 1010 per gram of tissue; from animal no. 2, 3.07 x 1010 per gram of tissue.
A cross-section at the middle of the right hind foot of animal no. 1 showed infiltration by numerous macrophages, many of which had a foamy cytoplasm (Fig. 2). There were few lymphocytes and plasma cells. The tendons of the dorsal foot were surrounded, infiltrated, and partly destroyed by the macrophages. A few nerves present showed perineural and intraneural inflammation and foamy degeneration of Schwann cells (Fig. 3). Acid-fast stain showed clumps of acidfast bacilli (AFB) inside macrophages, Schwann cells, and endothelial cells. It was interesting to note that although the granuloma was limited by the bone, tongues of inflammatory cells were seen to extend into the tissue between the bones. Most of the inflammatory tissue was confined to the dorsum of the foot above the level of the bony structures. The cross-section of the left hind foot pad of animal no. 2 showed a similar histopathological picture. The right inguinal node in animal no. 1 and the left inguinal node in animal no. 2 were invaded by collections of macrophages which replaced the germinal centers. Their cytoplasm was foamy and contained many AFB. The left inguinal node in animal no. -1 and the right inguinal node in animal no. 2 had scattered macrophages containing AFB.
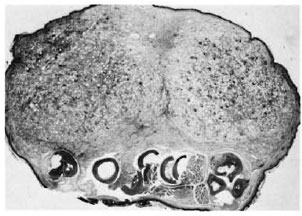
Fig. 2. Photomicrograph of a cross-section through the foot. Note large lepromatous nodule composed of foamy macrophages confined to the dorsum of the foot (H&E x 15).
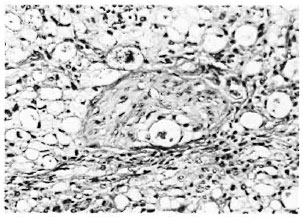
Fig. 3. Photomicrograph of a nerve in the lepromatous granuloma showing macrophages and Schwann cells containing M. leprae (Acid-fast stain3 x 260).
The ears of both animals showed scattered focal collections of macrophages containing AFB in the subepithelial tissue and around the cartilage infiltrating the perichondral tissue. The liver in both animals showed a few intracellular acid-fast organisms in scattered Kupffer cells. The spleen in animal no. 1 and the lungs in animal no. 2 showed scattered macrophages containing AFB. All other organs, such as the front paws, the skin of the back and abdomen, tail, eyelids, nose, heart, adrenals, kidneys, ovaries, uterus, stomach, small and large intestines, showed no significant lesion.
Two exhaustive and careful reviews of the literature on the mode of transmission of human leprosy have clearly shown that the most likely sites of entry are the skin and nasal mucosa (5,6).
In this study, convincing evidence is presented to show that in 2 of the 5 nude mice in which M. leprae were introduced through the abraded dorsal skin of the foot, a localized granuloma was produced at the site of entry. It was accompanied by infiltration of the inguinal lymph nodes by macrophages containing AFB followed by a generalized spread of the infection to the liver, spleen, ear, and lymph nodes. In our earlier study in which M. leprae were introduced into the nose, lungs, mouth, stomach, intact and abraded skin at the flanks, the infection took place only through the nasal mucosa. Lancaster later confirmed our findings on the entry of M. leprae through nasal mucosa but suggested that there might be a previous disease or damage to the nasal mucosa to facilitate the invasion by M. leprae (4). Our studies have shown that the nasal mucosa and the dorsal skin of the foot of the nude mice, both with a temperature around 30ºC, are the sites through which M. leprae have entered and eventually established generalized disease.
If athymic mice are a valid model of immunologically naive or M. leprae antigenspecific immunoincompetent humans, then it is reasonable to conclude that the two common sites of entry of M. leprae are the abraded skin, especially at the cooler sites of the body, and the nasal mucosa.
-Charles K. Job, M.D., F.R.C.Path.,
F.A.M.S.
Sumir K. Chehl, B.S.
Robert C. Hastings, M.D., Ph.D.
Laboratory Research Branch
G WL Hansen's Disease Center
Carville, Louisiana 70721, U.S.A.
Acknowledgments. This study was supported by grant #A122492 from the National Institutes of Health and Intra-Agency Agreement Y01-AI-5004 between the National Institutes of Allergy and Infectious Diseases and the Gillis W. Long Hansen's Disease Center. We are grateful to Mr. John Marcell for the technical work, Mr. Gregory McCormick for assistance in photographs, and Mrs. Mary M. Jackson for secretarial assistance.
REFERENCES
1. CHEHL, S., JOB, C. K. and HASTINGS, R. C. Transmission of leprosy in nude mice. Am. J. Trop. Med. Hyg. 34(1985)1161-1166.
2. CHEHL, S., RUBY, J., JOB, C. K. and HASTINGS, R. C. The growth of M. leprae in nude mice. Lepr. Rev. 54(1983)283-304.
3. JOB, C. K. and CHACKO, C. J. G. A modification of Fitc's stain for demonstration of M. leprae in tissue sections. Indian J. Lepr. 58(1986)17-18.
4. LANCASTER, R. D. The development and use of nude mouse as a model of lepromatous leprosy. Ph.D. thesis. University of London, 1985, pp. 220-236.
5. MACHIN, M. The mode of transmission of leprosy. In: Essays on Leprosy by Oxford Medical Students. Ryan, T. J. and McDougall, A. C, eds. Headington, Oxford: Department of Dermatology, The Slade Hospital, 1983, pp. 1-29.
6. PALLEN, M. J. and MCDERMOTT, R. D. How might Mycobacterium leprae enter the body? (Editorial) Lepr. Rev. 57(1986)289-297.
7. SHEPARD, C. C. Experimental leprosy. In: Leprosy. Hastings, R. C, ed. London: Churchill Livingstone, 1985, pp. 269-286.
Wayne M. Meyers, M.D., Ph.D., kindly served as Editor in regard to the submission, review, revision, and acceptance of this manuscript.