- Volume 58 , Number 2
- Page: 273–80
Controlled clinical trial of two multidrug regimens with and without rifampin in highly bacilliferous BL/LL south indian patients: a five-year report
ABSTRACT
A controlled clinical trial of two multidrug regimens in multibacillary lepromatous and near-lepromatous patients with a bacterial index (BI) of 2.5 or more was conducted. Patients were randomly allocated to either a two-drug regimen of dapsone plus clofazimine for 60 months or a four-drug regimen of rifampin, isoniazid, dapsone, and clofazimine for the first 3 months and clofazimine plus dapsone for the next 57 months. There was no difference between the rifampin and nonrifampin regimens with respect to the clinical improvement or bacteriological status of the patients at 60 months. Reactive states and neuritis were observed to be equal in the two patient groups.RÉSUMÉ
On a mené un essai clinique contrôlé de deux schémas thérapeutiques utilisant chacun plusieurs médicaments, chez, des malades atteints de lèpre multiba-cillaire et chez des malades proches du type lépromateux ayant un index bactériologique (BI) de 2, 5 ou davantage. Les malades ont été attribués au hasard à l'un ou l'autre de ces schémas thérapeutiques, l'un consistant en dapsone et clofazimine pour 60 mois, et l'autre comprenant quatre médicaments, à savoir la rifam-picine, l'isoniazide, la dapsone et la clofazimine pendant 3 mois, suivis de clofazimine accompagnée de dapsone pendant les 57 mois suivants. Aucune différence n'a été notée pendant les 60 mois du suivi entre les schémas thérapeutiques comprenant ou non de la rifampicine, pour ce qui est de l'amélioration clinique ou de l'état bactériologique de ces malades. La fréquence des états réactionnels ou des névrites fut égale dans les deux groupes de malades.RESUMEN
Se realizó un estudio clínico controlado de dos esquemas de tratamiento a base de drogas múltiples en pacientes multibacilares y en pacientes sublepromatosos con un índice bacteriológico (IB) de 2.5 ó más. Los pacientes fueron distribuidos (al azar) en un grupo que recibió un esquema de tratamiento con 2 drogas (dapsona más clofazimina por 60 meses) o en un grupo que recibió rifampina, isoniazida, dapsona y clofazi-mina por lo menos durante 3 meses y clofazimina más dapsona los siguientes 57 meses. No hubo diferencia entre los esquemas con y sin rifampina en cuanto a mejoría clínica o estado bacteriológico de los pacientes a los 60 meses. Los estados reaccionales y las neuritis fueron iguales en los 2 grupos de pacientes.For many years, the standard practice for the treatment of lepromatous leprosy was to prescribe dapsone (diaminodiphenylsul-fone, DDS) in a dosage of 5-10 mg/kg body weight/week for life. It is well known that many patients became increasingly irregular in taking drugs due to the long duration of treatment. Irregularity in treatment, together with low dosages (13,14,24,25), contributes to the emergence of sulfone resistance (1). Also, some patients develop clinical deterioration and continue to remain bacterio-logically positive despite prolonged dapsone monotherapy. The Mycobacterium leprae which these patients continue to harbor are probably partially or wholly sulfone resistant. Rifampin (RFP), a powerful bactericidal drug, was introduced in the treatment of multibacillary leprosy in the 1970s (10,21,27,28). However, even rifampin, given as monotherapy, is unable to produce faster clearance of bacilli (33). To prevent the emergence of drug resistance, multidrug therapy (MDT) was introduced in the treatment of leprosy. Rifampin has been tried in different combinations with other drugs (7,12,16,17,22,33,34), but there have been very few studies with a long-term follow up (6,15,23). Hence, a study was undertaken in lepromatous and near-lepromatous leprosy cases by the Tuberculosis Research Centre at Government Royapettah Hospital, Madras, India, to compare two regimens of MDT, with and without rifampin, with intensive follow up to 60 months.
MATERIALS AND METHODS
Patients admitted to the study were referred from the leprosy outpatient clinics in and around the city of Madras. A patient was eligible for the study if he/she was aged 12 years or more; the disease was classified clinically as polar lepromatous (LLp), subpolar lepromatous (LLs), or borderline lepromatous (BL); the bacterial index (BI) was 2.5+ or more on Ridley's scale, and he/she did not have renal, hepatic or cardiovascular disease, diabetes mellitus, epilepsy, or active tuberculosis.
The patients were randomly allocated to one of the following two regimens, after stratification according to the duration of previous antileprosy chemotherapy as less than 1 year, 1 to 5 years, and more than 5 years: a) RHCD (RFP) = Daily chemotherapy with rifampin 12 mg/kg body weight in three graded doses* according to body weight, plus isonia/id 300 mg, plus clofazimine 100 mg, plus dapsone 10 mg/kg body weight per week in three graded doses* for the first 3 months, followed by dapsone and clofazimine in the same dosages daily up to 60 months. b) CD (nonRFP) = Clofazimine 100 mg plus dapsone 10 mg/kg/week in three graded doses* daily up to 60 months.
The dosages were increased appropriately if the patient gained weight but were not reduced for weight loss. The patients attended the clinic daily for the first 3 months for supervised administration of drugs; subsequently, they attended twice a week for collection of drugs until 12 months, and once a week thereafter for the rest of the period. On the days of clinic attendance, the drugs were administered under supervision, and on the other days the drugs were to be self-administered.
Before starting treatment, all of the patients underwent a) a general physical examination, b) a clinical assessment of leprosy, c) an examination of skin smears from six active sites for leprosy bacilli to determine the bacterial index (BI) and morphological index (MI), d) estimations of hemoglobin and total and differential white blood cell counts, e) urine tests for albumin and sugar, f) estimations of total serum proteins, albumin/globulin (A/G) ratio, serum bilirubin, serum aspartate (AST) and alanine aminotransferase (ALT) activities and blood urea, g) Mitsuda lepromin test, h) tuberculin skin test using 1 TU PPD RT 23 with Tween 80, i) skin biopsy for histopathological examination, and j) color photographic transparencies of lesions. In addition, a clinical examination for leprosy was undertaken on admission by an independent assessor who was unaware of the treatment and the bacteriological findings.
During the treatment period, a clinical examination by a medical officer was done every month, examination of skin smears for BI and MI every 6 months from the same sites as on admission, blood and urine tests every 3 months up to 12 months and every 6 months thereafter, and biochemical investigations at 3, 6, 12 and 60 months. Skin biopsies were repeated at 6 and 60 months, and lepromin and tuberculin skin tests at 60 months; patients were seen by an independent assessor and color transparencies were taken every 6 months up to 36 months and every year thereafter.
Smears were taken from six skin sites: left and right earlobes, right eyebrow, and three other optional active sites. The smears were read independently by two experienced technicians. Whenever there was disagreement, an umpire reading was done.
RESULTS
Study population. In all. 210 (104 RFP, 106 nonRFP) patients were admitted to the trial. Of these, 33 (16 RFP, 17 nonRFP) have been excluded from the main analysis; 7 (3 RFP, 4 nonRFP) died due to causes other than leprosy; 10 (7 RFP, 3 nonRFP) migrated; 2 (both nonRFP) had received antituberculosis drugs; and 14 (6 RFP, 8 nonRFP) either absconded or discharged themselves against medical advice. The findings in the remaining 177 patients (88 RFP, 89 nonRFP) are presented here.
Prior to admission to the trial, 53 patients (60%) of the RFP group and 53 patients (60%) of the nonRFP group had received specific chemotherapy for less than 1 year; 25 (28%) and 24 (27%), respectively, for 15 years; 10(1 1%) and 12(13%). respectively, for more than 5 years. All but 22 of the 177 patients were male; the mean age was 28 years and the mean weight, 44.4 kg. Of 153 patients for whom histopathological reports were available, 120 (78%) were classified as lepromatous, 27 (18%) as borderline lepromatous, and 6 (4%) as indeterminate. However, 3 of these 6 indeterminate cases were clinically classified as lepromatous and 3 as borderline lepromatous by the independent assessor.
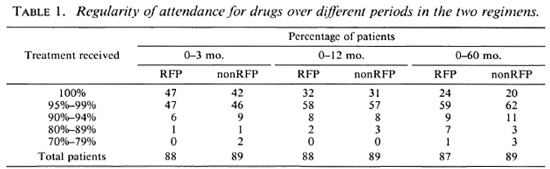
Drug regularity. During the first 3 months, 78 (44%) patients received every single dose of the drugs and 173 (98%) received 90% or more (Table 1) -a remarkable degree of regularity, considering that the patients were to attend daily for their drugs for supervised administration. Even over a 5-year period, more than 93% had collected 90% or more of their drugs. The regularity was similar in the two groups.
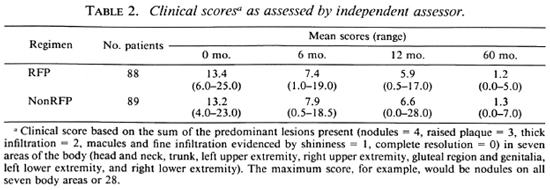
Clinical progress. Clinical progress was assessed by an independent assessor, who was unaware of the regimen or bacteriological results of the patients, using scores based on semiquantitative assessments. The body was divided into seven areas: a) head and neck, b) trunk, c) left and d) right upper extremities, e) gluteal region and genitalia, and f) left and g) right lower extremities. A clinical score was given for each area based on the predominant lesions present, as follows: nodules = 4; raised plaques = 3; thick infiltration = 2: macules and fine infiltration evidenced by shininess = 1; complete resolution = 0. The total of the seven areas represented the clinical status. The maximum score, for example, would be nodules on all seven body areas or 28.
The mean score on admission was 13.4 (range 6-25) for the RFP group and 13.2 (range 4-23) for the nonRFP group (Table 2). At 60 months, the average score was 1.2 and 1.3, respectively; both the fall and the rate of fall were similar in the two groups.
Progress was classified as: improvement, no change, or deterioration. Improvement was graded as marked, moderate or slight, depending on the total fall (25%-49% = slight, 50%-74% = moderate, > 75% = marked). In addition, sensory and motor assessments were done and recorded, including any paralysis.
At the end of 6 months, moderate or marked improvement was seen in 65% of the RFP group and 54% of the nonRFP group (p > 0.1). Corresponding figures at 60 months were very high -namely, 99% and 98%, respectively (Table 3), Again, the responses were similar in the two groups.

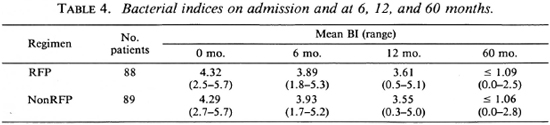
Bacteriological investigations. The mean bacterial index (BI) on admission was 4.32 in the RFP and 4.29 in the nonRFP groups. There was a steady fall in the BI value in both groups, and the reduction was similar for the two groups (Table 4). At 60 months, the BI values were 1.09 and 1.06, respectively.
Three patients in the RFP group and three patients in the nonRFP group had a BI of 0.0 at 60 months, but none had BI values of 0.0 for three consecutive months (Table 5); 31 patients in the RFP group and 37 in the nonRFP group had BI values between 0.1 and 0.9, and 46 and 37, respectively, had BI values between 1.0 and 1.9. None had a BI greater than 2.9.

The pretreatment morphological index (MI) was very low. Out of 177 patients, only 8% had an MI of 1.0% or more on admission and the maximum observed was 3.0%. No solid bacilli were seen in 88% in each group at 6 months, in 97% in the RFP group and 98% in the nonRFP group at 12 months, and in 100% in each group at 60 months.
Correlation of clinical score and BI. Although almost all of the patients in this study had shown moderate or marked clinical improvement over the 5-year period, the BI values at 60 months ranged from 0.0 to 2.8 (mean 1.07). The correlation coefficient between the two measures was 0.47 in the RFP group and 0.52 in the nonRFP group on admission and 0.02 and 0.2, respectively, at 60 months.
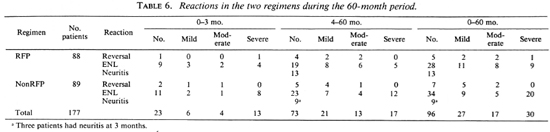
Complications. Of the 177 patients, 96 (46 RFP, 50 nonRFP) had reactions during the 60-month period (Table 6). These were classified as erythema nodosum leprosum (ENL) in 62 (20 mild, 13 moderate, 29 severe); as reversal reaction in 12 (5 RFP, 7 nonRFP); as neuritis in 22. In 23 (23%) of the 96 patients with reaction, the episodes occurred during the first 3 months. Severe reactions were noted in 10 patients in the RFP group and 20 patients in the nonRFP group (p = 0.1). The reactions were controlled with chloroquine, antihistamines, prednisolone and/or thalidomide, depending upon the severity.
During the 5-year period, 7 patients developed paralytic deformity of mild-to-moderate degree (4 belonged to the RFP group and 3 to the nonRFP group). All of these patients had experienced reactional episodes.
During the first 3 months, liver functions were monitored. The scrum bilirubin and scrum AST and ALT values were within normal limits for all patients on admission and at 3 months, except for one patient who had elevated values at 3 months but without clinical symptoms.
DISCUSSION
A controlled clinical trial was conducted in 177 patients with lepromatous or near-lepromatous leprosy, employing two regimens of chemotherapy, to elucidate the role of rifampin in multidrug therapy. The results up to the end of 5 years are reported here.
Clinical progress was recorded by way of body charting and the assigning of clinical scores. From this study, it appears that the scoring system used in this study, and as reported by Iyer, et al. (11), is a satisfactory method for assessing clinical progress.
The fall in BI in this study was approximately 0.6 log per year which is similar to that reported by Grosset (9). Of the 177 patients in this study, six patients had a PI value of 0.0 at 60 months. In the THELEP trials, 2 of 24 patients were reported to have a BI value of 0.0 in 41-53 months (30). The corresponding proportions reported by Ka-toch, et al. were 18 of 56 at 42 months and 10 of 24 at 48 months (15). In the study reported here, all of the patients had had a BI value of 2.5 or more on admission, compared with 4.0 or more in the other two studies.
Rifampin is a bactericidal drug and kills 99.9% of the organisms within a week (31).
Dapsone and clofazimine have been reported (19) to render the organisms nonviable in 90 days and 150 days, respectively. Isoniazid has been observed to have a potentiating effect in experimental therapy (5) and has been reported to show synergism with dapsone (2). In the present study, the bacteriological positivity continued to persist even after 5 years, as reported by others (18,26.29.33). From the results of this study, it appears that rifampin has not shortened the length of time necessary to achieve bacteriological negativity since the outcomes of the two regimens were similar. A long-term follow up of these patients is being undertaken after treatment to observe any relapses.
Leprosy patients are well known to be irregular in attending for treatment (20). El-lard (3) has reported that even among those attending treatment clinics as many as 30% actually self-administered less than 25% of their prescribed treatment. In the present study, the drugs were administered daily under supervision for the first 3 months, then supplied biweekly until 12 months, and then once a week until 60 months, the drugs being given under supervision on the days of attendance. Compliance of the patients was excellent in this study; thus, 44% received every single dose during the first 3 months. During the next 9 months when the patients were to attend twice a week, 90% collected 95% or more of their prescribed drugs. Attendance continued to be good for the rest of the 60-month period. All of the patients showed good clinical improvement, probably because of the high degree of regularity in attendance (4). However, 14 patients have been excluded from these analyses because they absconded or discharged themselves against medical advice.
Another interesting finding of this study is that reactions were neither more frequent nor their severity higher in the group which received rifampin during the first 3 months, contrary to the finding reported by Gro-enen, et al. (8). Indeed, in the present study, the incidence of severe reactions was higher in the nonrifampin group although the difference was not statistically significant (p = 0.1).
The success achieved by the nonrifampin regimen is a surprising and encouraging finding. All of the patients in this regimen had lepromatous leprosy, either LL or near-LL type. Forty percent of the 89 patients had already received more than 1 year of chemotherapy with dapsone prior to their admission to the study, and some of them could have had dapsone resistance at the time of admission to the trial. Even so, treatment with the nonrifampin regimen of clofazimine and dapsone for 60 months resulted in marked clinical improvement in 91% and moderate improvement in a further 6%. This was also reflected in a reduction of BI from 4.29 to 1.06. with an average annual fall of 0.6 log, similar to what has been reported for rifampin-containing regimens (9). The high efficacy of the regimen of clofazimine plus dapsone observed in this controlled clinical trial should be taken into consideration in planning national strategy for leprosy eradication.
Acknowledgments. The authors gratefully acknowledge the valuable contributions of the independent assessors. Dr. V. Ekambaram, Dr. K. Ramanujam, and Dr.G. Ramu.The invaluable assistance of Mrs. Lalitha Hari, Assistant Research Officer, and Mrs. S. Sivagama Sundari, Laboratory Technician (for reading the skin smears), Miss Therasa Xavier, Medical Social Worker, and the nursing staff is gratefully acknowledged. We are also grateful to Mrs. M. J. Nirmala for secretarial assistance.
REFERENCES
1. Browne, S. G. Dapsone-resislant Mycobacterium leprae in a patient receiving dapsone in low doses. Int. J. Lepr. 37(1969)296-301.
2. DHarmendra and Chatterjee, K. R. Isonicotin-ic acid hydrazide in the treatment of leprosy. Lepr. India 26(1954) 49-54.
3. Ellard, G. A. Combined treatment for lepro-matous leprosy. Lepr. Rev. 51(1980)199-205.
4. Ellard, G. A. , Gammon, P. T. and Harris, J. M. The application of urine test to monitor the regularity of dapsone self-administration. Lepr. Rev. 45(1974)224-234.
5. Freerksen, E. The technique of evaluating anti-leprosy medications at the Forschungsinstitut Bor-stel. Lepr. Rev. 46Suppl. (1975)25-39.
6. Ganapati, R. , Revankar, C. R. and Pai, R. R. Three years assessment of multidrug therapy in multibacillary leprosy cases. Indian J. Lepr. 59(1987)44-49.
7. Girdhar, B. K. and Desikan, K. V. " Pulsed" rifampicin therapy in leprosy. Lepr. India 51(1979)475-180.
8. Groenen, G. , Janssens, L. , Kayembe, T. , Nollet, E. , Coussens, L. and Pattyn, S. R. Prospective study on the relationship between intensive bactericidal therapy and leprosy reactions. Int. J. Lepr. 54(1986)236-244.
9. Grosset, J. H. Recent development in the field of multi-drug therapy and future research in chemotherapy of leprosy. Lepr. Rev. 57Suppl. 3(1986)223-234.
10. Holmes. I. R. and Hilson, G. R. F. The effect of rifampicin and dapsone on experimental Mycobacterium leprae infection: minimum inhibitory concentration and bactericidal action. J. Med. Microbiol. 5(1972)251-261.
11. Iyer, C. G. S. , Balakrishnan, S. and Ramu, G. A comparison of low and conventional dosages of dapsone in treatment of lepromatous leprosy. Lepr. India 49(1977)372-386.
12. Jopling, W. H. , Ridley, M. J. , Bonnici, E. and Depasquale, G. A follow-up investigation of the Malta Project. Lepr. Rev. 55(1984)247-253.
13. Karat, A. B. A. Low dose dapsone therapy in lepromatous leprosy. Lepr. Rev. 46Suppl.(1975)89-92.
14. Karat, A. B. A., Jeevaratnam, A. and Rao, P. S. S. An open trial of low doses of dapsone in the management of lepromatous leprosy. Lepr. Rev. 40(1969)99-105.
15. Katoch. K., Ramu, G., Ramanathan, U., Sengupta, U.. Sreevatsa, Sharma, V. D., Sm-vannavar, C. T. and Katoch, V. M. Results of a modified WHO regimen in highly bacilliferous BL/LL patients. Int. J. Lepr. 57(1989)451-457.
16. Koticha, K. K, Pade, S. S., Chulawala, R. G. and Juwatkar, P. S. Rifampicin (RFP) trial in lepromatous leprosy. Lepr. India 54(1982)441-447.
17. Languillon, J., Yawalkar, S. J. and Mc-Dougall, A. C. Therapeutic effects of adding Ri-mactane® (rifampicin) 450 milligrams daily or 1200 milligrams once monthly in a single dose to dapsone 50 milligrams daily in patients with lepromatous leprosy. Int. J. Lepr. 47(1979)37-43.
18. Leiker, D. L. Effect of mono treatment and combined treatment on the morphology of Myco. leprae in the skin. Lepr. Rev. 46Suppl.(1975)73-79.
19. Levy, L., Shepard, C. C. and Fasal, P. Killing of dapsone-rcsistant Mycobacterium leprae during treatment with B. 663. (Abstract) Int. J. Lepr. 37(1969)466.
20. Nair, N. G. K., Radhakrishna, S., Christian, M., Ramakrishnan, R. and Gopi, P. G. A 20-year study of the leprosy control programme at the Hemerijckx Leprosy Centre in Polambakkam in South India. Indian J. Lepr. 57(1985)562-574.
21. Pattyn, S. R. Comments on the chemotherapy of leprosy as influenced by present knowledge of Mycobacterium leprae. Lepr. Rev. 43(1972)126-136.
22. Pattyn, S. R., Rollier, M. T., Rollier, R., Saerens, E. J. and Dockx, P. A controlled clinical trial of continuous and intermittent rifampicin therapy during an initial three months period in lepromatous leprosy: final analysis. Lepr. Rev. 46Suppl.(1975)129-139.
23. Pattyn, S. R., Saint André, P., Ferraci, C. and Baquillon, G. Comparative study of two regimens of combined chemotherapy of one year duration in multibacillary leprosy; results after four and five years of follow-up. Int. J. Lepr. 52(1984)297-303.
24. Ramu, G. and Ramanujam, K. Lower dosage sul-phone regimen in leprosy. Lepr. India 37(1965)293-299.
25. Ramu, G. and Ramanujam, K. Low dose sul-phone treatment of leprosy. Lepr. India 41(1969)271-276.
26. Rees. R. J. W. Rifampicin; the investigation of bactericidal anti-leprosy drug. Lepr. Rev. 46Suppl.(1975)121-124.
27. Rees, R. J. W., Pearson, J. M. H. and Waters, M. F. R. Experimental and clinical studies on rifampicin in treatment of leprosy. Br. Med. J. 1(1970)89-92.
28. Rees, R. J. W., Waters, M. F. R., Helmy, H. S. and Pearson, J. M. H. The rate of kill of M. leprae in skin biopsies; comparison of dapsonc, streptomycin and rifampicin. (Abstract) Int. J. Lepr. 41(1973)682.
29. Rees, R. J. W., Waters, M. F. R., Pearson, J. M. H., Helmy, H. S. and Laing, A. B. G. Long term treatment of dapsone-resistant leprosy with rifampicin: clinical and bacteriological studies. Int. J. Lepr. 44(1976)159-169.
30. Seshadri, P. S. Controlled clinical trial of combined therapy in lepromatous leprosy. In: Proceedings of the Joint Meeting of Indian and Chemotherapy of Leprosy (THELEP) Scientists on Multidrug Therapy in Leprosy. Karigiri: Schieffe-lin Leprosy Research and Training Centre, 1988, pp. 21-24.
31.Shepard, C. C, Levy, L. and Fasal, P. Further experience with the rapid bactericidal effect of rifampicin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1124.
32.Subcommittee on Clinical Trials of the Chemotherapy of Leprosy (THELEP) Scientific Working Group of the UN DP/World Bank/ WHO Special Program for Research and Training in Tropical Diseases. THELEP controlled clinical trials in lepromatous leprosy. Lepr. Rev. 54 (1983) 167-176.
33.Waters, M. F. R., Rees, R. J. W., Pearson, J. M. H., Laing, A. B. G., Helmy, H. S. and Gelber, R. H. Rifampicin for lepromatous leprosy: nine years experience. Br. Med. J. 1(1978)133-136.
34.WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982, pp. 21-25. Tech. Rep. Ser. 675.
1. M.D., Dip.Lep.. Assistant Director; Tuberculosis Research Centre, Madras, India.
2. M.B.B.S., D.T.C.D., Research Officer; Tuberculosis Research Centre, Madras, India.
3. B.Sc, B.G.L., Technical Officer, Statistics; Tuberculosis Research Centre, Madras, India.
4. M.D., Director; Tuberculosis Research Centre, Madras, India.
5. B.S., Stat.Dip. (I.S.I.), Deputy Director - Statistics, Tuberculosis Research Centre, Madras, India.
6. M.D., F.A.M.S., Additional Director General, Indian Council of Medical Research, New Delhi, India.
7. (since deceased), M.B.B.S., D.T.M.&H., Dip. Epid. (Prague), Director, Schieffelin Leprosy Research and Training Centre, Karigiri, India.
Reprint requests to Dr. R. Prabhakar, Director. Tuberculosis Research Centre, Spurtank Road, Madras 600031, India.
Received for publication on 9 March 1989.
Accepted for publication in revised form on 13 December 1989.
* 20-29 kg = RFP 300 mg. DDS 50 mg; 30-44 kg = RFP 450 mg. DDS 75 mg; 45 kg or more = RFP 600 mg. DDS 100 mg.