- Volume 58 , Number 2
- Page: 281–95
Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatous leprosy
ABSTRACT
Twenty-one previously untreated lepro-matous patients were randomized into two groups and treated with either 800 nig pe-floxacin (PEFLO) or 400 mg ofloxacin (OFLO) once daily. The trial consisted of two parts: monotherapy f rom day 0 to day 56; and combined with the World Health Organization multidrug therapy (WHO/ MDT) regimen for multibacillary (MB) leprosy f rom day 57 to day 180. Four patients were removed f rom the trial because the organisms recovered f rom their pretreatment biopsies failed to infect mice. Among the remaining 17 cases, four (23.5%) had primary' resistance to dapsone but all of them were susceptible to rifampin. The initial (day 0) proportion of viable organisms, as measured by mouse foot pad inoculation, varied tremendously f rom patient to patient despite randomization during admission. Definite clinical improvement was noticed in virtually all patients after 22 doses of treatment with cither PEFLO or OFLO. A significant fall in the morphological index (MI) occurred as early as after 8 doses of PEFLO or after 22 doses of OFLO; the bacterial load also showed a moderate degree of reduction during the period of monotherapy. Although single-dose PEFLO or OFLO displayed only a modest degree of bactericidal effect against Mycobacterium leprae, about 99.9%, or 4 logs, of organisms viable on day 0 were killed by 22 doses of either PEFLO or OFLO. No significant difference in the therapeutic effect was detected between the two regimens. During PEFLO or OFLO monotherapy, except in one patient (case no. 10), the side effects were few and mild. Case no. 10 developed a psychic disorder after 27 days of PEFLO monotherapy, presumably due to the treatment with PEFLO. All of the patients tolerated the period of combined therapy extremely well, although some asymptomatic and transient laboratory abnormalities were observed. Because both PEFLO and OFLO displayed rapid bactericidal activities in human leprosy and were well tolerated by the patients, further clinical trials and field trials in evaluating the therapeutic effects of combined regimens containing both rifampin and PEFLO or OFLO are being organized. Since this is the first clinical trial in leprosy employing nude mice, in combination with normal mice, for monitoring the therapeutic effects of antimicrobials, the advantages, limitations and appropriate timing in using nude mice are discussed.RÉSUMÉ
Vingt et un malades lépromaleux non enore traités ont été répartis au hasard en deux groupes, dont l'un a été traité par 800 mg de pelloxacine (PFLX) par jour, en une prise, et l'autre par 400 mg d'ofloxacine (OFLX) au même rythme. L'essai a été conduit en deux phases : monothérapie du premier au 56 ème jour; et polychimiothérapie combinée selon la posologie recommandée par l'Organisation Mondiale de la Santé (OMS-PCT) pour les malades multibacillaires (MB), du 57ème au 180ème jour. Quatre malades ont été soustraits en cours d'essai, car les organismes recueillis dans les biopsies prélevées chez eux avant le traitement n'avaient pas infecté la souris. Parmi les 17 autres cas, 4 (23, 5%), présentaient une résistance primaire à la dapsone; tous ces malades étaient cependant susceptibles à la rifampicine. La proportion d'organismes viables au premier jour, telle qu'elle a pu être mesurée par l'inoculation dans le coussinet plantaire de la souris, a varié de manière considérable d'un malade à l'autre, et ceci malgré la répartition au hasard ayant présidé à l'essai. Une amélioration clinique prononcée a été observée chez pratiquement tous les malades après 22 doses de ce traitement, tant avec la PFLX qu'avec l'OFLX. Une chute significative de l'Index Morphologique (IM) est survenue dès la 8ème dose de PFLX, et dès la 22ème dose d'OFLX. La charge bactérienne a également diminué de façon modérée au cours de la monothérapie. Quoiqu'une dose unique de PFLX ou d'OFLX ne témoigne que d'effets bactéricides réduits contre Mycobacterium leprae, environ 99, 9% (4 logs) des organismes viables au début de l'essai avaient été tués par 22 doses de PFLX ou d'OFLX. Aucune différence significative dans les effets trérapeutiques n'a été notée entre ces deux schémas de traitement. Si ce n'est pour un seul malade (cas nš 10), les effets secondaires notés au cours de la monothérapic par PFLX ou OFLX étaient peu fréquents et bénins. Le cas n" 10 a développé un trouble psychique après 27 jours de monothérapie par PFLX, cette complication étant vraisemblablement due au traitement.Tous les malades ont extrêmement bien supporté la thérapeutique combinée, tout au long de la période où elle a été administrée, encore que quelques anomalies asymptômatiques décelées en laboratoire aient pu être observées. Puisque le PFLX comme l'OFLX témoignent d'une activité bactéricide rapide dans la lèpre humaine, et qu'ils sont bien tolérés par les malades, des essais complémentaires tant cliniques que sur le terrain sont prévus pour évaluer les effets thérapeutiques des schémas combinés contenant à la fois de la rifampicine et du PFLX ou de l'OFLX. Ceci étant le premier essai clinique dans la lèpre qui ait fait appel à la fois à la souris nue et à la souris normale, pour évaluer les effets thérapeutiques de médicaments anti-microbiens, on discute des avantages, des limitations, et du calendrier approprié pour l'utilisation des souris nues.
RESUMEN
Veintiún pacientes lepromatosos sin tratamiento fueron distribuidos al azar en dos grupos y tratados una vez al día con 800 mg de pefloxacina (PFLX) o con 400 mg de ofloxacina (OFLX). El ensayo consistió de dos partes: monoterapia del día 0 al 56, y adición de la terapia con múltiples drogas de la organización mundial de la salud (WHO/MDT) para lepra multi-bacilar (MB), del día 57 al día 180. Cuatro pacientes fueron eliminados del estudio porque los bacilos recuperados de sus biopsias previas al tratamiento fueron incapaces de infectar a ratones. De los 17 casos restantes, 4 (23.5%) tuvieron resistencia primaria a la dap-sona pero todos fueron susceptibles a la rifampina. La proporción inicial (día 0) de organismos viables (medida por la inoculación de la almohadilla del ratón), varió mucho de paciente a paciente no obstante su distribución al azar al inicio del estudio. En todos los pacientes se notó una definitiva mejoría clínica después del tratamiento con PFLX o con OFLX. Después de 8 dosis de PFLX o después de 22 dosis con OFLX ocurrió una significante caída en el índice morfológico; la carga bacteriana también mostró un grado moderado de reducción durante el período de monoterapia. Aunque las dosis únicas de PFLX u OFLX mostraron sólo un moderado efecto bactericida contra el Mycobacterium leprae, casi el 99.9%, o 4 logs, de los microorganismos vivos en el día 0 resultaron muertos después de 22 dosis de PFLX o de OFLX. No se detectaron diferencias significativas en el efecto terapéutico de los dos esquemas de tratamiento. Excepto en un paciente (caso no. 10), los efectos colaterales de la monoterapia con PFLX o con OFLX fueron pocos y moderados. El caso no. 10 desarrolló un desorden psíquico a los 27 días de monoterapia con PFLX probablemente debido a la droga. Todos los pacientes toleraron bien el período de terapia combinada, aunque se observaron algunas anormalidades asintomáticas y transitorias en los hallazgos de laboratorio. Debido a que tanto la PFLX como la OFLX mostraron rápidos efectos bactericidas en los pacientes con lepra y fueron bien tolerados por ellos, se están organizando esquemas de tratamiento combinando rifampina y PFLX o rifampina y OFLX. Puesto que este es el primer ensayo clínico en lepra donde además se usan ratones desnudos en combinación con ratones normales para evaluar los efectos terapéuticos de las drogas, se discuten las ventajas, las limitaciones y el tiempo más apropiado para el uso de los mismos.Pefloxacin (PEFLO) and ofloxacin (OFLO) are newly developed fluoroquinolone derivatives. Recent mouse experiments have demonstrated that both compounds display strong bactericidal activity against Mycobacterium leprae (8,9), and a preliminary clinical trial in previously untreated lepromatous leprosy patients has unequivocally proved the therapeutic effect of PEFLO in human leprosy (16). Because in the M. leprae -infected mouse foot pad system, OFLO in a daily dosage of 50 mg per kg displayed bactericidal activity similar to that of PEFLO administered in a daily dosage of 150 mg per kg, and because OFLO in a daily dosage of 150 mg per kg was fully bactericidal (8), a clinical trial in lepromatous leprosy was conducted. The trial was designed not only to compare more precisely the bactericidal activities of PEFLO 800 mg daily with that of OFLO 400 mg daily in human leprosy, but also to evaluate the side effects of PEFLO or OFLO alone and in combination with the World Health Organization/multidrug therapy (WHO/ MDT) regimen for multibacillary (MB) leprosy.
MATERIALS AND METHODS
Patients. Between September 1987 and April 1988, 21 previously untreated lepromatous patients were selected for the trial and hospitalized at the Institut Raoul Fol-lereau, Adzopé, Cote d'lvoire. During their first visit to the clinic, all of the patients had denied previous antileprosy treatment, and their urine dapsone analysis (10) was negative. Among the 21 leprosy patients: 19 were lepromatous (LL) and 2 were borderline lepromatous (BL) by clinical classification; 16 were male, 5 female aged between 16 and 61 (mean ± S.D. 32.6 ± 13.3) years old. None of them had a recent history of erythema nodosum leprosum (ENL). In order to recover higher numbers of M. leprae from their lesions for nude mouse inoculation, one of the criteria in selecting the patients was that the average bacterial index (BI) must be at least 4.0 before treatment. After admission, the patients were randomly allocated into two groups: 11 into the PEFLO group and 10 into the OFLO group.
Examinations at intake and during the trial. The examinations employed in the trial basically followed the THELEP Standard Protocol (18,23) and have already been described in the report of clinical trial of PEFLO (3). In brief, the following examinations were carried out in each patient: a thorough physical examination especially the examination for leprosy, photograph of skin lesions, lepromin test, PA chest X-ray, skin smears from six sites for the measurement of the BI, blood and urine analysis, liver function tests (SGPT, serum bilirubin and alkaline phosphatase), renal function (blood urea nitrogen) test, and two skin biopsies taken from separate active lesions which were large enough to provide several specimens which were shipped to Paris for histopathological examination and mouse foot pad inoculation. Of the two specimens, only the one with the higher logarithm10 of the number of M. leprae per mg of tissue (logAFB) and/or morphological index (MI) was used for mouse inoculation.
On days 7, 14, 28 and 56 during the trial, the patients were examined by the clinical investigator (notes were taken of any changes in the patients' lesions and the occurrence of leprosy reaction) and were interviewed for symptoms suggesting adverse reactions to PEFLO or OFLO. Blood and urine analysis, liver and renal function tests, and skin smears were repeated on day 28 and day 56; skin biopsy was repeated on days 7, 14, 28 and 56 from one of the two pretreatment biopsy sites which had been selected for mouse inoculation. The skin biopsies taken during the trial were for mouse inoculation only. During the period of combined therapy (from day 57 to day 180), the clinical and laboratory examinations, except skin biopsy, were repeated once monthly.
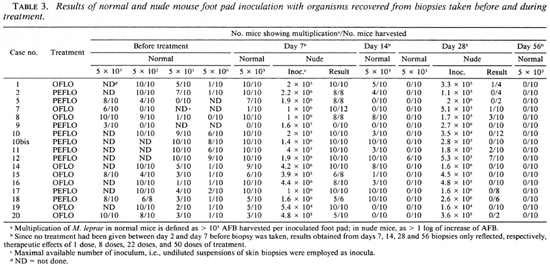
With respect to the mouse foot pad inoculations, there were two different purposes with the pretreatment specimens: a) to measure the susceptibility status of the organisms to dapsone and rifampin (RMP), and b) to determine the proportion of viable organisms before treatment. For those biopsies taken during the trial, the only purpose was to determine the proportion of viable organisms. Standard procedures (11,16) were followed for drug-susceptibility tests, and only immunologically intact (normal) mice were used. The procedures in measuring the proportion of viable organisms by inoculation into the foot pads of normal mice with serial diluted suspension have already been described (16). In the current trial, the organisms recovered from pretreatment (day 0) specimens were inoculated into normal mice with four different dilutions [5 x 103, 5 x 102, 5 x 101 and 5 x 100 acid-fast bacilli (AFB) per foot pad]; with organisms recovered from day 7 and day 28 specimens, one dilution (5 x 103) was inoculated into normal mice and one dilution (maximum available number of organisms, i.e., undiluted bacterial suspension) into congenital athymic (nude) mice; whereas only one dilution (5 x 103) was used in inoculating normal mice with organisms recovered from day 14 and day 56 specimens. Ten animals were inoculated per dilution, and the foot pads were harvested 12 months after inoculation. For measuring the proportion of viable organisms in pretreatment biopsies, the harvests were started cither from the group inoculated with 5 x 101 or 5 x 103 AFB per foot pad according to the already available results of harvests from the control group in drug susceptibility test, which were inoculated with the same organisms in the amount of 5 x 103 AFB per foot pad (16). When " 100% positivity" (i.e., multiplication of M. leprae was detected in all inoculated foot pads) or " 100% negativity" (i.e., no multiplication of M. leprae in any inoculated foot pads) were observed with a given dilution, no harvest was performed from the foot pads inoculated with higher concentrated inocula (in the former situation) or with higher diluted inocula (in the latter situation).
Treatment. PEFLO and OFLO were kindly supplied, respectively, by Laboratoires Roger Bellon (Paris, France) and from Laboratoires Diamant (Paris, France) as a gift. Both drugs were administered orally. The patients in both groups received treatment on day 1 with 800 mg of either PEFLO or OFLO, and stopped treatment from day 2 to day 7. The daily dose of 800 mg PEFLO or 400 mg OFLO was resumed on day 7 immediately after the biopsy was taken, and continued until day 56. From day 57 until day 180, on top of either PEFLO or OFLO as originally used for monotherapy, the WHO/MDT regimen for MB leprosy was added. In other words, patients received four drugs during that period: either PEFLO 800 mg once daily or OFLO 400 mg once daily plus RMP 600 mg and clofazimine (CLO) 300 mg once monthly and dapsone 100 mg and CLO 50 mg daily. All of the drugs were administered under supervision.
Statistical analysis. The data were analyzed by means of Student's t test for comparison of mean values by Fisher's exact probability test for comparison of median values and frequencies (19), and the reduction of viable organisms during treatment was tested by analysis of variance using the parallel line assay as described by Finney (5).
The proportions of viables were calculated and compared by the analysis of median infectious dose (ID50) (21). Because only one or two dilutions of organisms recovered from specimens taken during treatment have actually been inoculated, we have made the following assumptions for the calculation of ID50s. When multiplication of M. leprae hasbeen demonstrated in normal mice inoculated with 5 x 103 AFB per foot pad, we assumed that multiplication of M. leprae would have occurred in a similar proportion of mice inoculated with 5 x 102 AFB per foot pad, but no multiplication in mice inoculated with 5 x 101 AFB per foot pad. On the other hand, if no multiplication has been observed among inoculated mice, either in nude mice plus normal mice or in normal mice alone, we assumed that one mouse (nude mouse in the former case and normal mouse in the latter case) was positive, and the calculation results represent the maximal estimates for the proportion of viables. Taking the first patient (case no. 2) in Table 4 as an example, based on the actual observed results as shown in Table 3, the following assumptions were made for the calculations of ID50s: multiplication of M. leprae would have occurred in all of the 10 foot pads inoculated with 2 x 103 and 2 x 102 but in 0 of 10 foot pads inoculated with 2x101 organisms recovered from the biopsy taken on day 7; multiplication occurred in 4 of the 10 foot pads inoculated with 5 x 102 but in 0 of 10 foot pads inoculated with 5 x 101 organisms from the biopsy taken on day 14; multiplication occurred in 1 of the 4 nude mouse foot pads inoculated with 1.15 x 105 organisms from the biopsy taken on day 28; and multiplication occurred in 1 of the 10 foot pads inoculated with 5 x 103 organisms from the biopsy taken on day 56. However, despite the fact that the proportion of viables has been estimated from the results which showed multiplication in all inoculated normal mice with 5 x 103 AFB per foot pad, it is still possible that the proportion might be underestimated and, therefore, no statistical analysis on the changes of the proportion of the viables was performed under such circumstances. In addition, statistical analysis was also not carried out in cases where no absolute value was available from at least one of the two figures which were supposed to be compared. In summary, our principle is trying to avoid the exaggeration of killing of M. leprae by the treatment.
RESULTS
Characteristics of the patients before treatment. In 4 (19.0%) out of 21 patients, the organisms recovered from their pre-treatment biopsies failed to infect normal mice at the highest inoculum si/e (5 x 103 AFB per foot pad), indicating that the proportion of viable organisms before the trial must be very low. According to the protocol (18), these four patients were removed from the trial.
Among the 17 patients whose pretreatment organisms were able to infect mice, 4 (23.5%) were primary dapsone-resistant cases: 2 (nos. 19 and 20) low-degree, 1 (no. 10bis) intermediate-degree, and 1 (no. 1) high-degree resistance. The proportion of patients harboring dapsone-resistant M. leprae did not differ significantly between the two regimens (p = 0.21). All strains from the 17 patients were unable to multiply in mice treated with 10 mg/kg RMP once weekly by gavage, indicating that all of them were susceptible to RMP.
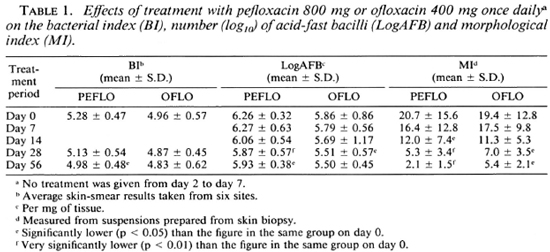
Twelve patients had an average BI of at least 5.0 and the logAFB of their pretreat-ment biopsy was at least 6.0, indicating that this group of patients had very high bacterial load before the trial (Table 1). Table 1 also shows that each patient had a high MI before treatment; actually the MI was more than 10% in 11 of them. None of these parameters was found to differ significantly between the two groups (p > 0.05).
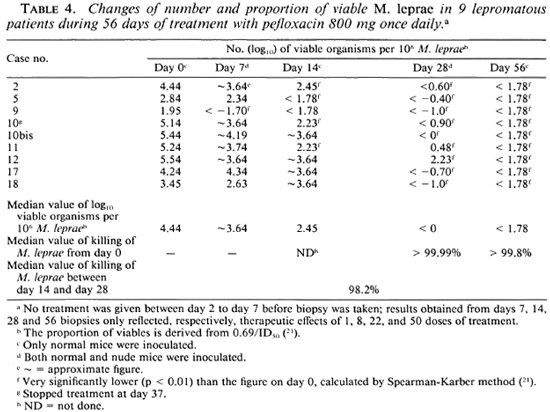
As shown in Tables 4 and 5, the proportion of viable organisms varied tremendously from patient to patient before treatment. Among the 17 cases, the average figure was 6.13 ± 10.65%(range 0.009% to 34.5%). Despite the randomization during admission, the proportion of viable M. leprae was 10.9 ± 13.3 in the PEFLO group and 0.79 ± 0.64 in the OFLO group, the mean proportion of viables being significantly higher in the PEFLO group than in the OFLO group (p < 0.05).
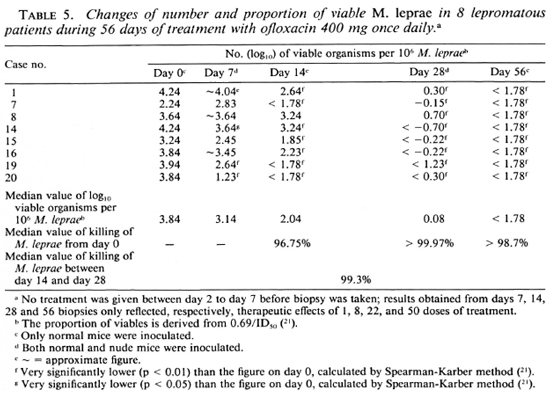
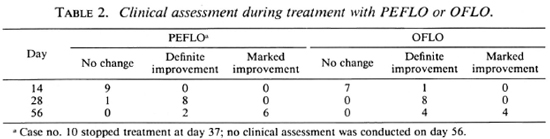
Clinical responses during the trial. Based on the changes of clinical manifestations during the trial, such as absorption of edema, amelioration of the nasal obstruction, regression of infiltration, and partial flattening of the nodules and plaques, the clinical responses are classified into the following three categories: a) no change, b) definite improvement, and c) marked improvement. As shown in Table 2, no change can be detected in 16 of 17 patients on day 14 (after 8 doses), definite improvement was reached in virtually all cases of both groups on day 28 (after 22 doses), and 6 of 8 cases in the PEFLO group and 4 of 8 cases in the OFLO group reached " marked improvement" on day 56 (Figs. 1 and 2). No significant difference can be detected on the distribution of various categories of clinical responses on days 14, 28 and 56 in both groups (p > 0.05). The clinical response continued during the period of combined therapy and very marked improvement had been observed in all patients at 6 months after starting the trial.
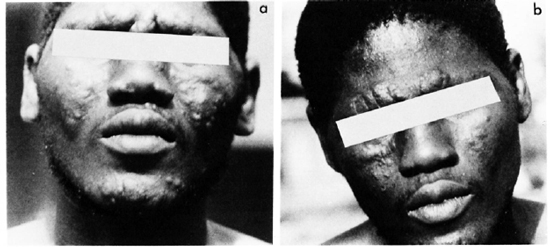
Fig. 1. Clinical manifestations of case no. 14: a) before treatment; b) marked improvement after 8 weeks of treatment with ofloxacin 400 mg once daily.
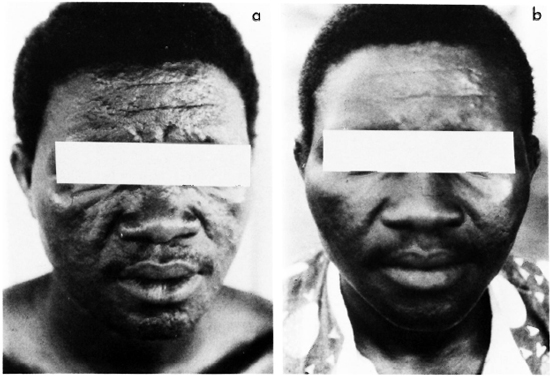
Fig. 2. Clinical manifestation of case no. 2: a) before treatment; b) marked improvement after 8 weeks of treatment with pefloxacin 800 mg once daily.
Neither ENL nor reversal reaction was observed during the trial, including the period of monotherapy by PEFLO or OFLO and the period of combined therapy with either PEFLO or OFLO and WHO/MDT.
The changes in the BI, logAFB and MI during the trial are presented in Table 1. As expected, these parameters gradually declined during the course of treatment. After 56 days of treatment, the BI had decreased by 0.30 ± 0.27 unit in the PEFLO group and by 0.13 ± 0.55 unit in the OFLO group. The reduction of the BI did not differ significantly between the two groups (p > 0.05). On day 56, the logAFB had reduced by 0.33 ± 0.34 in the PEFLO group and by 0.36 ± 0.70 in the OFLO group; again, no significant difference was observed between the two groups (p > 0.05). As usual, the fall of the MI during treatment was more rapid than that of the BI and logAFB. The mean MI at day 14 of the PEFLO group and at day 28 of the OFLO group was already significantly lower than that of day 0 (p < 0.05). Comparing the two groups, the fall of the MI from day 0 to day 56 was significantly more in the PEFLO group than that in the OFLO group (p < 0.05). On day 56, the mean MI of the PEFLO group was very significantly lower than that of the OFLO group (p < 0.001).
Measurement of bactericidal activities against M. leprae by the treatments. The mouse foot pad inoculation results are presented in Table 3, and the proportion of viable organisms before and during treatment with PEFLO or OFLO are presented in Table 4 and 5, respectively.
The organisms recovered from biopsies taken on day 7 multiplied in a majority of the inoculated normal mice, indicating that the loss of viability resulting from a single dose of PEFLO or OFLO was limited. A significant reduction of the proportion of viable organisms was observed only in 1 (case no. 9) out of 9 patients in the PEFLO group and in 3 (case nos. 14,19 and 20) out of 8 patients in the OFLO group, and no significant difference has been observed on the frequency of such patients between the two groups (p > 0.05). There were 9 patients whose organisms recovered from day 7 biopsies multiplied in all inoculated normal mice (" 100% positivity" ). Seven of these 9 patients derived from 8 patients whose initial (day 0) proportion of viables was more than 1.0%. The frequency of " 100% positivity" of multiplication of M. leprae on day 7 was significantly higher in those patients whose initial proportion of viables was more than 1% than in those of less than 1% (p = 0.012), indicating that the multiplication in normal mice with M. leprae recovered on day 7 was correlated with the initial (day 0) proportion of viables of the same strains.
As compared with day 0, a significant reduction of viable organisms was observed in 4 cases of the PEFLO group and in 7 cases of the OFLO group on day 14. The frequency of such patients did not differ significantly between the two groups (p > 0.05). Therefore, one to two " logs" killing of M. leprae had already occurred in more than 50% of the patients after 8 daily doses of treatment with either PEFLO or OFLO. However, 12 out of the 17 strains still multiplied, or were positive, in normal mice at various proportions; 8 of them came from the 8 patients whose initial (day 0) proportions of viables were more than 1.0%, and 4 of them derived from the 9 patients whose initial proportions were less than 1.0%. By Fisher's exact probability test, significantly more proportion of positivity was observed in strains with initial proportion of viables more than 1.0% than in strains of less than 1.0% (p = 0.020).
As shown in Table 3, with organisms recovered on day 28, multiplication in normal mice inoculated with 5 x 103 organisms was observed in only one strain (case no. 12, who had the highest proportion, 34.5%, of viables before treatment) and five strains (case nos. 1, 7, 8, 11 and 12) showed partial multiplication in nude mice inoculated with organisms ranging from 1.7 x 105 to 5.3 x 105 AFB per foot pad. The results in Tables 4 and 5 demonstrated that a very significant reduction of viable organisms had occurred in all of the patients. After 22 daily doses of either PEFLO or OFLO, the killing rate of M. leprae rangedfrom 99% to > 99.999%. There was virtually no difference in the killing rate between the two groups; the median value was > 99.99% in the PEFLO group and > 99.97% in the OFLO group. In comparing the results obtained from biopsies taken on day 14 and on day 28, additional significant killing of M. leprae has been observed in all cases for which figures were comparable. The median value of the killing by the additional 14 daily doses was 98.2% in the PEFLO group and 99.3% in the OFLO group; again, no significant difference can be observed between the two groups (p > 0.05).
On day 56, 0 of the 17 strains multiplied in normal mice inoculated with 5 x 103 organisms per foot pad. The median value of the killing rate during the period was > 99.8 in the PEFLO group and > 98.7 in the OFLO group; again, no statistical difference can be detected between the two groups (p > 0.05).
Figure 3 summarizes the reduction of viable organisms from day 0 to day 28. The data for day 7 were excluded because the single dose displayed only a very modest effect. The analysis of the data was based on a factorial experiment (dose response as factor 1 and compound as factor 2), with a parallel line assay (5). The deviation to linearity was not significant as was the deviation to parallelism. The regression was found highly significant [F(l,45) = 163.47, p < 0.001]; whereas no statistical difference was found between PEFLO and OFLO. Therefore, all of the data could be summarized in a single regression equation. It is clearly demonstrated that about 4 logs of M. leprae had been killed by 22 doses of either PEFLO or OFLO treatment.
Fig. 3. Evolution of the number of viable M. leprae in 106 AFB recovered from skin biopsies of patients treated with pefloxacin (◊) and ofloxacin ( ).
).
Side effects of the treatments. Except for patient no. 10, the PEFLO or OFLO treatments were well tolerated in all patients including those who had been removed from the trial because the organisms recovered from their pretreatment biopsies failed to infect mice. Two patients, one in each group, complained of nausea after the first few doses of treatment; one case in the OFLO group felt dizziness after the first dose only; another patient in the OFLO group had diarrhea during the first 4 weeks of treatment. All of the complaints were mild, and none of them led to withdrawal of the treatment.
Patient no. 10 was a 61-year-old female LLp patient. She started PEFLO treatment on 2 November 1987. On 29 November, 27 days after starting PEFLO treatment, she suddenly developed serious insomnia, manias and persecution delusion. From 2 December, she had received the tranquilizer Largactil (chloropromazine) 25 mg intramuscularly once daily, and the symptoms gradually subsided within 4-5 days. She stopped PEFLO on 9 December (after 30 doses of treatment) but continued Largactil until 11 December. On 15 December, 4 days after stopping Largactil, her psychic disorders recurred and were gradually being controlled again by intramuscular injections of 25 mg Largactil and oral administration of 6 mg Lexomil (bromazepam) once daily. The symptoms did not recur after her treatment was changed to WHO/MDT on 16 January 1988. Neither she nor her family had any history of mental disorder.
All of the patients tolerated extremely well the combined therapy containing PEFLO or OFLO and the three drugs of the WHO/ MDT regimen for MB leprosy. No patient complained of any symptoms suggesting an adverse reaction caused by the treatment.
The following observations were obtained during monotherapy with PEFLO or OFLO among the laboratory parameters monitored: elevation of serum bilirubin (case no. 1), alkaline phosphatase (case nos. 1, 5 and 8). and blood urea nitrogen (case no. 5). Neither elevation of SGPT nor hematological abnormalities were seen. During the period of combined therapy, the changes included: elevation of alkaline phosphatase (case nos. 1, 2, 7, 8, 16), of serum bilirubin (case nos. 7, 8, 14). of SGPT (case no. 19), of urea nitrogen (case nos. 1, 10). anemia and thrombocytopenia (case no. 2). However, all of these changes were mild in severity and transient in nature, without association with clinical symptoms. Several patients had elevated alkaline phosphatase (case nos. 10, 11, 14) or elevated SGPT (case nos. 11, 16) and elevated urea nitrogen (case nos. 10, 11) at the beginning of treatment, but these parameters gradually returned to normal or remained unchanged during the course of treatment.
DISCUSSION
Although the assessment of clinical improvement in leprosy is very much subjective and difficult to quantify, definite clinical improvement was observed in virtually all patients after 28 days, or 22 doses, of treatment with either PEFLO 800 mg or OFLO 400 mg. Further improvement was noticed as the treatment continued and was more obvious among those patients who had more severe infiltration, nodules, plaques and histoid lepromas before treatment. Our impression is that the clinical improvement during PEFLO or OFLO treatment occurred earlier than that during dapsone or clofazimine monotherapy. A significant reduction in the MI was observed as early as after 14 days (8 doses) of PEFLO treatment, and after 28 days (22 doses) of OFLO treatment. The bacterial load, in terms of the BI and logAFB, also showed a moderate degree of reduction. The most important finding is the direct demonstration of rapid bactericidal effects as measured by serial mouse foot pad inoculation. About 99.99%, or 4 logs, of viable organisms at day 0 were killed by 22 doses of either PEFLO or OFLO treatment. The results of the PEFLO group in the current trial are identical with what has been described in the pilot trial of PEFLO (16). In summary, the 56 days of monotherapy with 800 mg once daily of PEFLO or 400 mg once daily of OFLO demonstrated a very promising therapeutic effect in human leprosy, and virtually no significant difference was observed between these two regimens.
Very little information related to the side effects of long-term fluoroquinolone treatment is available (22). Despite the fact that the side effects were mild in the first clinical trial with 6-month PEFLO, because the sample size was small, we appealed to the investigators not to ignore the possibility of serious side effects caused by fluoroquinolones in clinical trials of leprosy (16). The current trial, with 21 patients, again demonstrated that the side effects caused by PEFLO or OFLO were, in general, few and mild. However, one patient (case no. 10) did show a psychic disorder after 27 days of PEFLO treatment, and according to her history, the psychic disorder might be attributed as being a side effect of PEFLO. It was reported that the frequency of central nervous system side effects differs from one fluoroquinolone to another, and the frequency for OFLO (1.0%) was very similar to PEFLO (1.1%) (12). Nevertheless, psychic troubles were very rare and were only observed in elderly patients treated with PEFLO (3). Case no. 10 did not take theophylline during the trial, therefore her psychic trouble being caused by an interaction between theophylline and fluoroquinolone can be ruled out. She was 61, the eldest patient in the trial. A high blood level of PEFLO might be responsible for her psychic disorder because it has been reported that the peak concentration (Cmax) of fluoroquinolones, including PEFLO, was much higher in elderly than in younger subjects (14). With respect to OFLO, adverse central nervous system reactions seem to be at an increased rate in the elderly (14). Therefore, both PEFLO and OFLO should be used cautiously in elderly patients. Although up to now we have not observed cutaneous photosensitivity after treating about 30 patients with PEFLO or OFLO, the possibility must be borne in mind because the majority of leprosy patients are in tropical sunny areas and photosensitivity occurs more frequently with PEFLO than with other fluoroquinolones (3).
In the future, PEFLO or OFLO will definitely not be used alone for the treatment of leprosy. It is important to know whether or not side effects will be more serious when PEFLO or OFLO is combined with other antileprosy drugs. The current trial clearly demonstrates that the patients tolerated extremely well the combined therapy which consisted of PEFLO/OFLO, RMP, dapsone and clofazimine; the occurrence of asymptomatic laboratory abnormalities during the period of combined therapy was neither more frequent nor more serious than either PEFLO/OFLO monotherapy or WHO/ MDT. Therefore, the combination of PEFLO/OFLO with other antileprosy drugs does not seem to increase the risk of toxicity.
Serial mouse foot pad inoculations have been applied as the most efficient technique for monitoring the chemotherapeutic effects of antimicrobials in leprosy since 1964 (20), not very long after the mouse foot pad technique was developed. However, up to now, immunological intact (normal) mice have been used in the great majority of these studies (20), and they can barely measure a killing rate of more than 2 logs because of the limited inoculum size (5 x 103 to 1 x 104 per foot pad) as clearly demonstrated in our pilot PEFLO trial (16) and the current trial. Among the immunologically compromised rodents, only thymectomized-irra-diated (TR) mice (17) and neonatally thy-mectomized rat (NTR) (4) have been employed in a limited number of trials (6,7,24,26,27), but to the best of our knowledge, the nude mouse has never been systematically applied in any clinical trial of leprosy although it is extraordinarily susceptible to infection with M. leprae (15) and has been available for leprosy research since 1976 (1,13). In addition, to date, except for our earlier trial of PEFLO (16), only a single inoculum was prepared from each biopsy for foot pad inoculation, and the negativity of multiplication during treatment merely indicates that the number of viables in the inocula had been reduced to an undetectable level by the treatment but the killing or reduction of the proportion of viables cannot be quantified. Although the technique of measuring the proportion of viable organisms in samples by inoculation with serial 10-fold diluted inocula was developed more than a decade ago (2), it has only been applied to quantify the bactericidal activities of antimicrobials in experimental chemotherapy research.
In order to obtain more precise information for comparing the bactericidal activities of PEFLO and OFLO in human leprosy, we inoculated both normal and nude mice with different dilutions of bacterial suspensions prepared from biopsies taken on several occasions. Based on the results obtained from the trial, the advantages of these approaches can be summarized as: a) inoculating four different dilutions from day 0 biopsies into normal mice provided a relatively precise baseline information for quantifying the changes in the proportion of viables by the treatments and b) the combination of normal and nude mice inoculations was able to measure the proportion of viable organisms down to about 0.0001 % to 0.000002%, which is much lower than the level in using normal mice alone (no less than 0.006%) and thereby significantly increased the sensitivity in measuring the killing rate up to 4 or even 5 logs, depending upon the proportion of viables in the day 0 samples and the maximum available number of organisms for nude mice inoculation.
However, despite the fact that the nude mouse is extraordinarily susceptible to M. leprae , the current trial also demonstrated the limitation of the nude mouse in detecting a tiny fraction of viable organisms in untreated lepromatous patients. The limitation is mainly due to: a) as shown in Table 1, on average no more than 106 organisms per mg of tissue can be recovered from a biopsy even in advanced untreated lepromatous patients and b) the small size of the nude mouse foot pad severely limits the volume of inoculum. Therefore, as shown in Table 3, despite the fact that the great majority of the patients had very high bacterial loads before treatment, among the 34 nude mice inoculations only one inoculum reached the 107 level; 15 inocula each were at the level of 106 or 105 AFB per foot pad, and only 3 were at the level of 104. No multiplication in nude mice inoculated with 5 x 105 or 5 x 106 AFB per foot pad indicates that the proportion of viables is < 0.00006% or < 0.000006%, respectively. Assuming that the proportion of viables before treatment was 10%, > 99.999% or > 99.9999% of killing might be demonstrated. Because lepromatous patients may have 1010 to 1011 viable organisms, at best we are only able to measure the initial 5 to 6 logs of the killing even using nude mice. Therefore, more sensitive techniques for measuring the further killing of M. leprae should be developed.
Since this was the first clinical trial monitoring the therapeutic effects by both normal and nude mouse inoculations, lessons, especially the timing in inoculating nude mice, should also be learned. It is evident that the nude mice cannot, and also should not, be applied as frequently as normal mice because they are expensive in terms of purchase and maintenance. The design of the trial included the evaluation of the therapeutic effects of single-dose PEFLO and OFLO, because the pilot trial of PEFLO had demonstrated a very significant killing of M. leprae by the first biopsy taken 2 months after starting treatment (16). Although both compounds displayed strong bactericidal activities in mice (8,9), they were not as bactericidal as RMP. In retrospect, the inclusion of the nude mouse inoculation after a single dose of PEFLO or OFLO was unnecessary, and normal mice should have been inoculated with the same dilutions of inocula on day 0. It seems reasonable that only normal mice were inoculated with organisms from specimens taken on day 14 (after 8 doses) but it might be more informative if one (5 x 102) or two (5 x 102 and 5 x 101) additional dilutions were included. The inclusion of nude mice inoculation with specimens from day 28 was appropriate since the 4 to 5 logs killing could not be measured otherwise. The major deficit of the design was that only normal mice were inoculated with samples taken at the end of the trial, i.e., day 56, and therefore it was impossible to evaluate the possible additional killing effects from day 28 to day 56. In summary, although it is difficult to appropriately plan the timing of nude mouse inoculations in a clinical trial based on the results of animal experiments, it appears that nude mice should be used only at the stage of treatment when the proportion of viable organisms has been reduced to a level below that detectable by normal mice inoculation, and after which the inoculation of normal mice alone is no longer informative.
Unlike RMP, single-dose PEFLO or OFLO showed only a modest degree of therapeutic effect in human leprosy, therefore neither compound can be administered once monthly. However, the demonstration that about 4 logs of organisms were killed by only 22 doses of PEFLO or OFLO has very significant clinical relevance. In theory, all the spontaneous drug-resistant mutants must be eliminated before MDT is stopped. With respect to the current WHO/MDT regimen for MB leprosy, the mutants resistant to either dapsone or clofazimine will be killed rapidly by RMP because of its powerful bactericidal activity; whereas the mutants resistant to RMP can only be killed by clofazimine and dapsone. Unfortunately, both drugs display only a weak bactericidal effect and the elimination of RMP-resistant mutants is bound to be slow. Up to now, no precise information is available about the frequency of RMP-resistant mutants; they are probably in the same rate, about 10-7, as in M. tuberculosis (25). Assuming there are 1011 to 1012 M. leprae in an untreated lepromatous patient and about 10% of the organisms are viable, the total number of viable organisms is about 1010 to 1011, and about 103 to 104 of these viables are resistant to RMP. By definition, the 3 to 4 logs RMP-resistant mutants should respond to other antimicrobials as RMP-susceptible organisms. There is good reason to expect that the combination of either PEFLO or OFLO with RMP will greatly accelerate the elimination of RMP-resistant mutants and therefore be able to shorten the duration of MDT. However, the assumption should be confirmed by clinical research. Further clinical trials and field trials along this direction are being organized.
In the clinical trials of dapsone and clofazimine, a large difference in the length of treatment required to reach negativity of foot pad inoculation was observed between different patients and no satisfactory explanation was available (20). As a by-product of the trial, the analysis suggests that the most likely explanation is that the proportion of viable organisms varied tremendously before treatment; the higher the proportion of viables on day 0, the longer the period of treatment required to reach negativity of foot pad inoculation.
Acknowledgments. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. We thank Mr. G. Hejblum and Dr. C. Ricca for their assistance in statistical analysis. We also wish to express our appreciation to the Laboratoires Roger Bellon, Paris, for supplying pefloxacin and to the Laboratoires Diamant, Paris, for supplying ofloxacin.
REFERENCES
1. Colston, M. J. and Hilson, G. R. F. Growth of Mycobacterium leprae and M. marinum in con-genitally athymic (nude) mice. Nature 262(1976) 399-401.
2. Colston, M. J., Hilson, G. R. F. and Banerjee, D. K. The " proportional bactericidal test," a method for assessing bactericidal activities of drugs against Mycobacterium leprae in mice. Lepr. Rev. 49(1978)7-15.
3. Desplaces, N., Gutmann, L., Carlet, J., Guibert, J. and Acar, J. F. The new quinolones and their combinations with other agents for therapy of severe infections. J. Antimicrob. Chemother. 17Suppl. A(1986)25-39.
4. Fieldsteel, A. H. and Levy, L. Neonatally thy-mectomized Lewis rats infected with Mycobacterium leprae: response to primary infection, secondary challenge and large inocula. Infect. Immun. 14(1976)736-741.
5. Finney, D. J. Parallel line assays. In: Statistical Method in Biological Assay. 2nd cd. Finney, D. J., ed., London: Charles Griffin & Co. Ltd., 1964, pp. 99-136.
6. Gelber, R. H., Humphres, R. C. and Fieldsteel, A. H. Superiority of the neonatally thymecto-mized Lewis rat (NTLR) to monitor a clinical trial in lepromatous leprosy of the two regimens of rifampin and dapsone. Int. J. Lepr. 54(1986)273-283.
7. Gelber, R. H. and Levy, L. Detection of persisting Mycobacterium leprae byinoculation of the neonatally thymectomized rat. Int. J. Lepr. 55(1987)872-878.
8. Grosset, J. H., Guelpa-Lauras, C. C, Perani, E. G. and Beoletto, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
9. Guelpa-Lauras, C. C, Perani, E. G., Giroir, A. M. and Grosset, J. H. Activities of pefioxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
10. Huikeshoven, H. and Madarang, M. G. Spot test for detection of dapsone in urine: an assessment of its validity and interpretation in monitoring dapsone self-administration. Int. J. Lepr. 54(1986)21-24.
11. Ji, B. Drug susceptibility testing of Mycobacterium leprae. Int. J. Lepr. 55Suppl.(1987)830-836.
12. Kitzes-Cohen. R. Quinolones in CNS infections; pharmacokinetics. Quinolones Bull. 3(1987)11-14.
13. Kohsaka, K. . . Mori. T. and Ito, T. Lepromatoid lesion developed in the nude mouse inoculated with Mycobacterium leprae-animal transmission of leprosy. Lepro 45(1976)177-183.
14. LeBel, M. Pharmacokinetic behaviour of fluoroquinolones in the elderly. Quinolones Bull. 4( 1988)9-11.
15. McDermott-Lancaster, R. D., Ito, T., Kohsaka, K., Guelpa-Lauras, C. C. and Grosset, J. H. Multiplication of Mycobacterium leprae in the nude mouse, and some applications of nude mice to experimental leprosy. Int. J. Lepr. 55Suppl.(1987)889-895.
16. N'Deli. L., Guelpa-Lauras, C. C, Perani, E. G. and Grosset, J. H. Effectiveness of Pefloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)12-18.
17. Rees. R. J. VV. Enhanced susceptibility of thymec-lomized and irradiated mice to infection with Mycobacterium leprae. Nature 211(1966)657-658.
18. Report of the First Meeting of THELEP Scientific Working Group. Annex I. Standard protocol for chemotherapy trials in lepromatous leprosy. TDR/SWG-THELEP(1)/77. 3.
19. Sachs, L. Applied Statistics. A Handbook of Techniques. 2nd ed. . New York-Berlin-Heidelberg-Tokyo: Springer-Verlag, 1978.
20. Shepard, C. C. A brief review of experiences with short-term clinical trials monitored by mouse-footpad inoculation. Lepr. Rev. 52(1981)299-308.
21. Shepard, C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae. Int. J. Lepr. 50(1982)96-101.
22. Smith, C. R. The adverse effects of fluoroquinolones. J. Antimicrob. Chemother. 19(1987)709-711.
23. Subcommittee on Clinical Trials of the Chemotherapy (THELEP) Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. THELEP controlled clinical trials in lepromatous leprosy. Lepr. Rev. 54(1983)167-176.
24. Subcommittee on Clinical Trials of the Chemotherapy (THELEP) Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
25. Trnka, L. Rifampicin (RMP). In: Antituberculosis Drugs. Bartmenn, K., ed., Berlin-Heidelberg-New York-London-Paris-Tokyo: Springer-Verlag, 1988, pp. 205-225.
26. Waters, M. F. R. . Rees, R. J. W., McDougall, A. C. and Weddell, A. G. M. Ten years of dapsone in lepromatous leprosy: clinical, bacteriological and histological assessment and the finding of viable leprosy bacilli. Lepr. Rev. 45(1974) 288-298.
27. Waters, M. F. R. . Rees, R. J. W., Pearson, J. M. H., Laing. A. B. G., Helmy, H. S. and Gelber, R. H. Rifampicin for lepromatous leprosy: nine years' experience. Br. Med. J. 1(1978)133-136.
1. M.D., Professor; Bactériologie et Virologie, Faculté de Médecine Pitié-Salpêtrière, 91 Blvd. de l'Hôpital, 75634 Paris Cedex 13, France.
2. M.D.; Bactériologie et Virologie, Faculté de Médecine Pitié-Salpêtrière, 91 Blvd. de l'Hôpital, 75634 Paris Cedex 13, France.
3. M.D., Assistant; Bactériologie et Virologie, Faculté de Médecine Pitié-Salpêtrière, 91 Blvd. de l'Hôpital, 75634 Paris Cedex 13, France.
4. Technical Officer, Bactériologie et Virologie, Faculté de Médecine Pitié-Salpêtrière, 91 Blvd. de l'Hôpital, 75634 Paris Cedex 13, France.
5. M.D., Director, Institut Raoul Follereau, Adzopé, Côte d'Ivoire.
Received for publication on 1 September 1989.
Accepted for publication in revised form on 9 January 1990.