- Volume 58 , Number 2
- Page: 296–301
Selection of MDT strategies through epidemiometric modeling
ABSTRACT
The epidemiometric model of leprosy, built on Polambakkam, India, data, is used to compare the impact on incidence of dapsone and different multidrug therapy (MDT) strategies. The simulations show that generalization of MDT could have a dramatic impact on transmission of the disease. Relapses after MDT, although important f rom an individual point of view, have a negligible influence on the incidence. Introduction of MDT requires investments that, during the first few years of the program, are much greater than for dapsone monotherapy. These arc, however, rapidly absorbed due to the rapidly declining number of new cases, particularly when MDT is not limited to multibacillary cases but is administered to all patients.RÉSUMÉ
On a utilisé le modèle épidémiométrique de la lèpre développé à partir des données recueillies à Polambakkam, en Inde, pour comparer les effets sur l'incidence de stratégies thérapeutiques basées respectivement sur la monothérapie à la dapsone et sur différents schémas de polychimiothérapie. Les simulations ont montré que la généralisation de la polychimiothérapie devraient avoir un effet radical sur la transmission de la maladie. Les récidives après polychimiothérapie, aussi importantes soient-elles au point de vue du malade, n'ont qu'une influence négligeable sur l'incidence. L'introduction de la polychimiothérapie exige, au cours des premières années du programme, des investissements qui sont beaucoup plus importants que ceux requis par la monothérapic à la dapsone. Ces dépenses sont toutefois rapidement compensées par le déclin rapide du nombre de nouveaux cas, particulièrement lorsque la polychimiothérapie n'est pas limitée aux cas multibacillaires, mais est administrée à l'ensemble des malades.RESUMEN
Se usó el modelo epidemiométrico de la lepra construido con los datos de Polambakkan, India, para comparar el impacto de incidencia de las estrategias de tratamiento con dapsona y con múltiples drogas (MD). Las simulaciones muestran que la generalización del tratamiento con MD podría tener un impacto dramático sobre la transmisión de la enfermedad. Las recaídas después del tratamiento con MD, aunque importantes desde un punto de vista individual, tienen una influencia insignificante sobre la incidencia. La introducción de un tratamiento con MD requiere inversiones mucho mayores durante los primeros años del programa que la monoterapia con dapsona. Estas son, sin embargo, rápidamente absorbidas debido a la rápida disminución en el número de casos nuevos, particularmente cuando el tratamiento con MD no se limita a los casos multibacilares sino que es adminís-tardo a todos los pacientes.The emergence of sulfone-resistant strains of Mycobacterium leprae, and the rapid spread of primary resistance, have made multidrug therapy (MDT) the backbone of leprosy control. While secondary resistance to dapsone is a worldwide phenomenon, the proportion among treated multibacillary (MB) leprosy patients reaching up to 40% in some areas, primary dapsone resistance has also been widely detected, sometimes with proportions up to 70% among the newly detected patients (14). Implementation of MDT is therefore the first priority today in terms of leprosy control. If this is not done the results obtained over the last three decades in leprosy control could in the long term be nullified. Computer simulations have shown that with an annual incidence rate of 3% of secondary dapsone resistance, a rate that has already been observed in some countries (17), the gradual decline in incidence observed with dapsone monotherapy could be reversed within a few years (5).
According to a recent survey (3), 34% of the cases registered for treatment worldwide are reported to be undergoing MDT. Large differences in coverage are, however, observed between regions, ranging from 12% for Africa to 39% for South-East Asia. An insufficient perception of the urgency of the matter may explain the low coverage. Other factors are the operational and financial constraints associated with the introduction of MDT.
The selection of target groups to be treated by priority with MDT has also been subject to controversy. In the case of limited resources or restricted supplies, should the treatment be administered by priority to the MB patients, while dapsone monotherapy is continued in the paucibacillary (PB) cases until more resources become available? In addition, it is presumed that none of the existing drugs, used alone or in combination, affects the persisters, that is, fully drug-sensitive M. leprae that arc able to survive for many years in MB patients despite the presence of bactericidal concentrations of the drugs (11). In THELEP-supported trials, pcrsisters have been detected in about 9% of the biopsy specimens from rifampin-treated patients (10). The relation between persisters and the occurrence of relapses has yet to be elucidated. If persisters may lead to relapse, then persisters could possibly reduce in the long term the decrease in incidence expected with MDT.
The objectives of this study are thus to simulate different therapeutic strategies with an epidemiometric model in order to: a) compare trends of incidence after dapsone monotherapy and MDT, given either exclusively to MB cases or to all the leprosy patients; b) predict the possible impact of pcrsisters on incidence, and c) make a cost-effectiveness comparison of the different therapeutic strategies cited above.
MATERIALS AND METHODS
The parameters required to build up the model were calculated from the individual data collected on the 35,200 patients detected in Polambakkam, South India, between 1955 and 1970. The structure of the model and its basic assumptions have been described elsewhere (5,6). The parameters of the model relevant to this study are: a) in Polambakkam more than 85% of the new patients arc of the PB type and b) an estimated 75% of the patients are detected within 1 year after onset; delay between onset and detection follows a negative exponential distribution. The specific assumptions for MDT are:
One dose of MDT kills enough bacilli to make the patient noninfectious, while the same process takes 1 year with dapsone monotherapy. Treatment is, however, given for 2 years to the MB and 1 year to the PB patients (according to the structure of the model, transitions from one state to another are only possible on an annual basis).
Emergence of drug resistance is restricted to the patients treated with dapsone monotherapy.
Relapse cases after completion of MDT are still fully sensitive to it, or to at least one drug in the combination.
Microbial persistence is limited to MB leprosy and is always followed by relapse.
Relapses after MDT take place after a 5-ycar latency period, and are uniformly distributed between the sixth and tenth year after treatment was stopped.
For the cost-effectiveness analysis, only the costs of the drugs were taken into account. These were calculated on the basis of the prices made for the ILEP associations, supposing treatment with dapsone monotherapy takes 3 years for PB and 10 years for MB cases.
Total cost of treatment is committed since its inception:

RESULTS
The computer simulations of the trends in incidence rates with dapsone monotherapy and MDT, cither exclusively for the MB patients or for all leprosy patients, are shown in Figure 1. A 50% reduction of incidence is obtained after some 8 years with dapsone monotherapy. The same result is achieved in 5 years if MDT is given to MB cases and in less than 4 years if MDT is given to all patients.
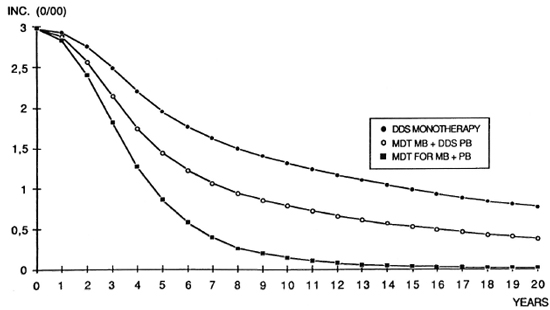
Fig. 1. Computer simulation of incidence of leprosy with three different therapeutic regimens.
In view of the apparent inability of MDT to get rid of all persisters, simulations have been made to predict the possible impact of different relapse rates on incidence. The results of these simulations arc shown in Figure 2. Even very high and unrealistic relapse rates have only a relatively weak impact on incidence and never result in cancelling the effects of MDT.
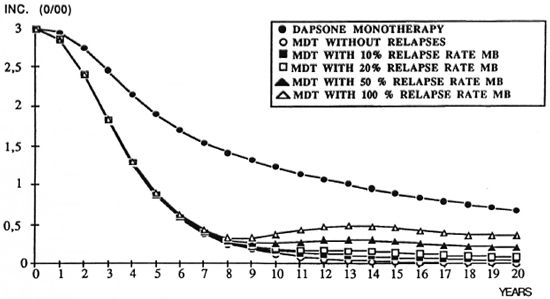
Fig. 2. Computer simulation of leprosy incidence with dapsone and MDT with different relapse rates.
Figure 3 shows the trend of the annual cost of each therapeutic regimen. Introduction of MDT requires important additional investments at the beginning of the program compared with dapsone monotherapy. However, an equilibrium between the annual costs of MDT and monotherapy is reached after some 20 years if MDT is given to MB cases exclusively, and after 3 years if MDT is given to all leprosy patients.
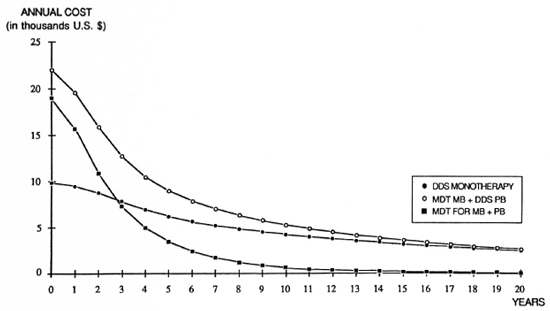
Fig. 3. Annual cost of drugs for three different therapeutic regimens (simulations made for 1,000,000 population).
Figure 4 shows the cumulative cost of each therapeutic strategy. MDT given to MB cases only requires an investment which, even after 20 years, remains higher than that of dapsone. If MDT is given to all leprosy cases, the total cumulative cost is similar after 8 ½ years (notwithstanding the fact that by that time the predicted incidence is only 17% and 26%, respectively, of what it would have been with dapsone and with MDT given only to MB cases) (Fig. 1). Compared to the cost of dapsone monotherapy, the total cost of the drugs necessary to obtain a 50% reduction in the incidence would be 39% higher if MDT is given to the MB cases only, but 13% lower if it is given to all the leprosy patients.
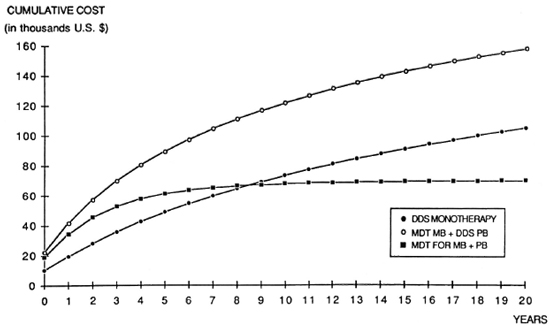
Fig. 4. Cumulative cost of drugs for three different therapeutic regimens (simulations made for 1,000,000 population).
DISCUSSION
The simulations made for this study show that the introduction of MDT could lead to a sharp decline of the leprosy incidence rate. This is confirmed by observations made in Karigiri, South India (8). At the moment, while MDT is used in many endemic countries, its use is often restricted to only a limited number of patients, usually MB cases by priority, arguing of their higher infectious capacity. It is thus interesting to note that the model predicts a much sharper decline of incidence if MDT is given to all leprosy patients rather than limited to MB cases. Of course, this can only be verified if, as in Polambakkam. the proportion of MB cases among the new patients is low.
According to present knowledge, it seems that relapse rates after using the WHO-recommended MDT regimens arc very low (2,4,9,12,13). However, it is still too early to give precise estimates of the risk. As a consequence, since relapses would present a serious problem both for the patients and from an operational point of view, some countries faced with a shortage of resources could be tempted to take a conservative view and concentrate their efforts by treating a limited number of patients with MDT until complete negativation or even longer, rather than treating all MDT patients for 24 months only. The model shows that as long as relapses are not more frequent than with dapsone monotherapy, a most unlikely possibility, their effect on incidence is negligible. It is therefore recommended that, within a given set of resources, all MB leprosy patients be treated with MDT for 24 months, even with a risk of relapse, rather than treating a limited number for a longer period. This, however, requires that an efficient surveillance system should be established to detect relapses as early as possible.
The problems raised by the introduction of MDT are manyfold. Some arc operational or organizational; others arc financial. The purchase of drugs represents only part of the total investment required for the implementation of MDT. Other investments, for training, purchase of equipment or vehicles, or running a laboratory, for instance, were not considered since they are too dependent on the local pre-existing situation. A comparison between leprosy control projects should take into consideration these local variables. However, limiting this study to the cost of the drugs, it is clear that the investment required by the generalization of MDT to all leprosy patients, higher at the beginning of the program, decreases rapidly to become less than that required by dapsone monotherapy. The main reason is a much sharper decline of incidence. Moreover, except for the drugs, the investment is more or less the same, whether MDT is limited to MB cases or is given to all leprosy patients. The simulations show the obvious advantages of the latter strategy from economical and public health points of view.
CONCLUSIONS
The introduction of MDT has become necessary because of the emergence and rapid spread of dapsone resistance. In spite of more important operational constraints and investments, MDT could result in a dramatic reduction of the leprosy incidence. In view of the often high proportion of pau-cibacillary cases, MDT should be administered to all leprosy patients and not be limited to multibacillary cases.
Acknowledgments. This study was supported by the Amici di Raoul Follercau, Association Française Raoul Follereau, Damicn Foundation Belgium, Fondation Luxembourgeoise Raoul Follereau, and Le Secours aux Lépreux Canada (Member-Associations of ILEP). The assistance of Mrs. F. Bertrand in reviewing this paper is gratefully acknowledged.
REFERENCES
1. Baquillon, G. . Ferracci, C, Van Loo, G. and Pattyn, S. R. Further results on dapsone-resistant leprosy in Bamako. Lepr. Rev. 54(1983)19-21.
2. Benjamin-Huntley, R. and Rose, P. WHO multidrug therapy-a review of 956 domiciliary patients starting MDT between December 1981 and December 1987. (Abstract) Int. J. Lepr. 57Suppl.(1989)335.
3. Department of Epidemiology, Catholic University of Louvain, Brussels. Global evaluation of the introduction of multidrug therapy. Lepr. Epidemiol. Bull. 2(1989).
4. Ji, B. -H., Grosset, J. H. and Noordeen, S. K. Progress in chemotherapy research under THE-LEP programme. (Abstract) Int. J. Lepr. 57Suppl.(1989)336.
5. Lechat, M. F., Misson, C. B., Lambert, A., Bouckaert, A., Vanderveken, M. and Vellut, C. Simulation of vaccination and resistance in leprosy using an epidemiometric model. Int. J. Lepr. 53(1985)461-467.
6. Lechat, M. F., Misson, J. Y., Vellut, C. M., Misson, C. B. and Bouckaert, A. Un modèle épidémiométrique de la lèpre. Bull. WHO 51(1974)361-373.
7. Pearson, J. M. H., Haile, G. S. and Barnetson, R. St. C. Dapsone-resistant leprosy in Ethiopia. Lepr. Rev. 50(1979)183-199.
8. Rao, P. S. S. . Jesudasan, K., Pannikar, V. K. and Christian, M. Epidemiological impact of multidrug therapy in Gudiyatham control area, Karigiri. (Abstract) Int. J. Lepr. 57Suppl.(1989)331.
9. Sansarricq, H. Technical problems related to multidrug therapy in leprosy control. (Abstract) Int. J. Lepr. 57Suppl.(1989)324.
10. Subcommittee on Clinical Trials of the Chemotherapy of Leprosy (THELEP) Scientific Working Group of the UNDP/World Bank/ WHO Special Programme for Research and Training in Tropical Diseases. The THELEP controlled clinical drug trials. Int. J. Lepr. 55(1987)864-871.
11. Toman. K. Bacterial persistence in leprosy. Int. J. Lepr. 49(1981)205-217.
12. Van Brakel, W., Kist, P., Noble, S. and O'Toole, L. Relapses after multidrug therapy for leprosy: a preliminary report of 22 cases in West Nepal. Lepr. Rev. 60(1989)45-50.
13. Vijayakumaran, P., Pannikar, V. K., Jesudasan, K. and Christian, M. Field trials of combined therapy in lepromatous leprosy. (Abstract) Int. J. Lepr. 57Suppl.(1989)337.
14. WHO Expert Committee on Leprosy. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
1. M. D., D. P. H., Head; Department of Epidemiology and Preventive Medicine, School of Public Health, Catholic University of Louvain, Clos Chapelle-aux-Champs 30, 1200 Bruxelles, Belgium.
2. M. D.; Department of Epidemiology and Preventive Medicine, School of Public Health, Catholic University of Louvain, Clos Chapelle-aux-Champs 30, 1200 Bruxelles, Belgium.
3. Research Associate, Department of Epidemiology and Preventive Medicine, School of Public Health, Catholic University of Louvain, Clos Chapelle-aux-Champs 30, 1200 Bruxelles, Belgium.
4. M. D., Hemerijckx Government Leprosy Centre, Polambakkam 603309, Tamil Nadu, India.
Received for publication on 29 August 1989.
Accepted for publication on 17 November 1989.