- Volume 58 , Number 2
- Page: 302–10
Immunologic defects in leprosy patients. I. Evidence of immune aberration of suppressor-T lymphocytes in lepromatous leprosy
ABSTRACT
Immunoregulation in various types of leprosy patients was evaluated in vitro using peripheral blood mononuclear leukocytes (PBML) stimulated with phytohemagglu-tinin-P (PHA-P) or concanavalin A (ConA) for a cell-mediated immune (CMI) assay or pokeweed mitogen (PWM) for a humoral-mediated immune (HMI) assay. The immune responses were evaluated by a lymphocyte transformation test (LTT) and lymphocyte-mediated cytotoxicity (LMC) for the immunoregulation of CMI, and a reverse hemolytic plaque assay for measuring the plaque-forming cells (PFC) and a sandwich ELISA for measuring IgG concentrations for the immunoregulation of HMI.In LTT with PHA-P or ConA, the mean of the normal controls was not significantly different f rom the means of the untreated LL, BL, BB, BT, and TT leprosy patients. However, a wide variation of LTT results f rom BT to LL patients was noted: the LTT results of TT patients and normal controls were less variable. A similar pattern of immune responses was noted when studied by LMC in untreated LL, BL, BB, BT, and TT leprosy patients and normal controls. When the untreated patients and normal controls were studied for PFC, using PBML stimulated with PWM, a very similar pattern of PFC was obtained with the different types of leprosy patients.
The immunoregulatory role of lymphocytes in leprosy patients was further evaluated by cell mixing cultures. ConA-stim-ulated PBML f rom lepromatous leprosy patients were mixed with normal PBML and then stimulated with PHA-P. The immune regulation was then measured by LMC. Untreated BL/LL patients having a bacterial index (BI) of 3+ or more had significantly less suppressive activity than treated BL/ LL patients having a BI of less than 3 + , less than treated TT patients, and less than normal controls.
The activity of suppressor-T lymphocytes f rom untreated LL patients was further evaluated by the isolation of CD8+ T cells or ConA-sheep erythrocyte (SRBC) rosetted T cells f rom PBML of these patients and normal controls. The CD8+ T cells or ConA-SRBC rosetted T cells were cultured in various percentages with normal PBML and then stimulated with PWM. The immunoregulation of these T-cell populations was measured by quantitative determination of the PFC, using a reverse hemolytic plaque assay, or by quantitation of IgG with an ELISA. The CD8+ T cells and ConA-SRBC rosetted T cells f rom these LL patients showed significantly less suppressive activities in all of the tested cell concentrations when compared with normal controls.
RÉSUMÉ
En vue d'évaluer in vitro l'immunorégulation dans diflérents types de la maladie chez des sujets atteints de lèpre, on a eu recours à deux sortes de titrages, l'un basé sur la réaction des leucocytes mononucléaires du sang périphériue (PBML) à la stimulation par la phy-tohémagglutinine-P (PHA-P), ou par la concanavaline A (Con A) pour tester l'immunité à médiation cellulaire (CMI), l'autre basé sur la réaction de ces mêmes leucocytes stimulés par l'extrait mitogène de Phytolacca americana, (PWM), pour explorer la réponse immunitaire humorale (HMI). La réaction immunitaire portant sur la régulation de l'immunité à médiation cellulaire a été jugée par l'épreuve de transformation lymphocytaire (LTT) et par l'épreuve de cytotoxteité à médiation lymphocytaire (LMC); la régulation de l'immunité humorale a été mesurée par dosage par page dereflethémolytique inverse (PFC), ainsi que par une épreuve ELISA à double couches mesurant la concentration des IgG.Avec l'épreuve de transformation lymphocytaire stimulée par la PHA-P ou la ConA, la moyenne observée chez des témoins normaux n'était pas significativement differente des moyennes relevées chez les malades de la lèpre non traités atteints des types LL, BB, BT, et TT. Toutefois, on a enrigistré une grande variation des résultats de cette épreuve entre les sujets BT et LL. En effet, les résultats de l'épreuve de transformation lymphocytaire chez les malades TT et chez les témoins normaux étaient moins variables. Un profil similaire des réponses immunitaires a été observé à l'épreuve de transformation lymphocytaire chez des malades de la lèpre LL. BL. BB, BT, TT et chez des témoins normaux. Lorsque le PFC était étudié chez les cas non traités et chez les témoins normaux, en utilisant des leucocytes mononucléaires du sang périphérique stimulés par le PWM, on a obtenu un profil très similaire pour l'ensemble des malades quel que soit leur type de lèpre.
Le rôle immunorégulateur des lymphocytes chez, les malades de la lèpre a alors été évalué en mêlant des cultures de cellules. Les leucocytes mononucléaires obtenus chez des patients atteints de lèpre lépromateuse stimulés par la ConA ont été mélangés avec des leucocytes mononucléaires normaux, et stimulés ensuite par la PHA-P. La régulation immunitaire a été ensuite mesurée par l'épreuve de transformation lymphocytaire. Les malades non traités atteints de lèpre BL/LL, ayant un index bactériologique (BI) de 3+ ou davantage, présentaient une activité suppressive significativement inférieure à celle des sujets BL/LL traités ayant un BI inférieur à 3 + , et également une activité inférieure à celle des malades TT traités et des témoins normaux.
On a ensuite évalué l'activité des lymphocytes T-suppresseurs, recueillis chez des malades LL non traités, en isolant les cellules T CD8+ ou les cellules T entraînant la formation de rosettes d'erythrocytes de mouton après stimulation par la ConA (SRBC), parmi les leucocytes mononucléaires de ces malades et de témoins. Les cellules T CD8+ et les cellules T SRBC ont été cultivées dans des milieux contenant diverses proportions de leucocytes mononucléaires normaux, puis stimulées par le PWM. L'immunorégulation de ces populations de cellules T a été mesurée par la détermination quantitative du PFC, en utilisant un dosage par plage de reflet hémolytique inverse, et par la quantification des IgG au moyen d'une épreuve ELISA. Les cellules T CD8 + et les cellules T en rosette ConA-SRBC provenant de ces malades LL ont témoigné d'une activité suppressive moins significative à toutes les concentrations de cellules utilisées que celle notée chez des témoins normaux.
RESUMEN
Se evaluó, in vitro, la inmunoregulación en pacientes con varios tipos de lepra, usando sus leucocitos mo-nonucleares de sangre periférica (LMN) estimulados con fitohemaglutinina P (FHA-P), con concanavalina A (Con-A) o con fitolaca americana (PWM). La inmunorcgulación de la respuesta inmune celular (IC) se valoró usando una prueba de transformación de lin-focitos (PTL) y una prueba de citotoxicidad mediada por linfocitos (CML); la inmunorcgulación de la respuesta humoral se valoró por cuantificación del número de células formadoras de placa (CFP) en un ensayo de placas hemolíticas reversas y porcuantificación de IgG usando un ensayo enzimático (ELISA).En la PTL con FHA-P o con Con-A, el resultado promedio en los controles normales fue similar al encontrado en los pacientes LL, BL, BB, BT y TT no tratados. Sin embargo, los resultados de la PTL en los pacientes de los grupos BT al LL mostraron una amplia variación. Los resultados de la PTL en los pacientes TT y en los controles normales fueron menos variables. En el ensayo de CML se encontró un patrón muy similar de respuesta en los controles sanos y en los pacientes LL, BL, BB, BT y TT. También se obtuvo un patrón muy similar de respuesta entre los controles sanos y los pacientes con los diferentes tipos de lepra cuando sus LMN se estimularon con PWM para inducir la formación de CFP.
Para el ensayo por cultivo mixto de células, los LMN de los pacientes LL estimulados con Con-A se mezclaron con LMN de individuos sanos y después se estimularon con FHA-P, entonces se midió la regulación inmune por CML. Los pacientes BL/LL sin tratar y con un indice bacteriano (IB) de 3+ o más, tuvieron menor actividad supresora que los pacientes BL/LL tratados con un IB menor de 3 + , que los pacientes TT tratados, y que los controles sanos.
También se evaluó la actividad de los linfocitos T supresores en los pacientes LL y en los controles sanos aislando los linfocitos CD8+ y cuantificando las células formadoras de rosetas con eritrocitos de carnero acoplados a Con-A. Las células CD8+ o las células T separadas por la formación de rosetas con GRC-Con-A, se cultivaron en varios procentajes con LMN normales y después se estimularon con PWM. La inmu-norcgulación de estas células T se midió cuantificando las CFP por medio de un ensayo de placas hemolíticas reversas o por cuantificación de IgG por ELISA. Las células T CD8+ y las células T separadas por rosetas con GRC-Con-A de los pacientes LL, mostraron una actividad supresora significativamente menor que los controles sanos a todas las concentraciones celulares probadas.
The immunological unresponsiveness of lepromatous leprosy patients to Mycobacterium leprae has interesting implications in the study of immunoregulation in infectious diseases. Leprosy is an infectious disease with a diversity of clinical manifestations and a histopathological classification that correlates well with its immunological status (2,20). In tuberculoid leprosy, there are few acid-fast bacilli (AFB) in the tissues, and this type of patient develops high levels of cell-mediated immunity (CMI). In lepromatous leprosy, there arc extraordinary numbers of AFB in the tissues, and the patients have very low levels of CMI (9).
There are many hypotheses explaining the unresponsiveness of lepromatous leprosy patients to in vivo skin tests or in vitro CMI tests. The older view was that lepromatous leprosy patients had generalized ancrgy for CMI responses (4,25). Later, it was shown that in the vast majority of patients with lepromatous leprosy, there is good responsiveness to a variety of recall antigens, e.g., purified protein derivative (PPD) (17,18,24). Suppression of the immune response by suppressor-T cells or monocytes of lepromatous leprosy patients has been demonstrated (15,16). It was shown later that the unique M. leprae antigen, phenolic glyco-lipid-I, is capable of inducing suppression of mitogenic responses of lepromatous leprosy patients' peripheral blood mononuclear leukocytes (PBML) in vitro, and this provided evidence that the suppressor cells recognize the specific terminal trisaccharide moiety (14). However, others have not been able to find disease-related suppression in lepromatous leprosy (1,6,19,23). Recently, the leprosy-specific nonresponsiveness in vitro has been shown to result from a lack of interlcukin 2 (IL-2) production which can be overcome by the addition of exogenous IL-2 (7). However, after reassessment of a large group of leprosy patients for M. leprae T-cell proliferative response, it was concluded that lepromatous leprosy patients were either entirely anergic or very weakly positive, and IL-2 enhanced only a few of the weakly positive response patients (10). The immunologic defect(s) of CMI in leprosy patients, therefore, is not as yet fully understood.
We attempted to investigate aberrant immunoregulation in lepromatous leprosy using PBML or T-cell subpopulations from various types of leprosy patients compared with normal controls.
MATERIALS AND METHODS
Leprosy patients and normal controls. All leprosy patients were seen at the McKean Rehabilitation Center in Chiang Mai, Thailand. They were diagnosed by the Ridley and Jopling classification based on clinical and histopathological evidence (20). Untreated leprosy patients were defined as new cases or those treated with dapsone (DDS) for not more than 6 weeks. Treated leprosy patients were cases treated with antileprosy drugs for more than 6 weeks. Normal controls were volunteer students and laboratory personnel.
Preparation of peripheral blood mononuclear leukocytes (PBML). Heparinized venous blood was obtained from normal controls and leprosy patients. PBML were prepared by the standard Ficoll-Hypaque density centrifugation (3). PBML were washed three times with RPMI 1640 medium (GIBCO, Grand Island, New York, U.S.A.) and adjusted to a concentration of 1 x 106 cells/ml in RPMI 1640, supplemented with 10% fetal bovine scrum (FBS; GIBCO). The RPMI 1640 medium always contained 100 units of penicillin and 100 μg of streptomycin per ml.
ConA activation of PBML. Fifty micrograms of concanavalin A (ConA; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) in 50 μl RPMI medium was added to 4 x 106 PBML in 4 ml of medium in a sterile plastic tube (Falcon, Cockeysville, Pennsylvania, U.S.A.) and then incubated for 1618 hr in a 5% CO2 incubator. The ConA-activated and nonactivated PBML were washed with RPMI medium, and 4 ml of fresh 10% FBS in RPMI medium was added to each tube. The cell suspensions were cultivated for a total period of 60 hr, then washed twice with 0.3 M α-methyl-D mannoside (Sigma) in RPMI medium.
Separation of T cells. T cells from ConA-activated and nonactivated PBML were separated by adding 0.1 ml of 1% AET-SRBC (2-aminoethylisothiouronium-hy-drobromide treated sheep red blood cells) (8) to 1 x 106 PBML, incubated at 37°C for 10 min, and then centrifuged at 600 x g x 2 min and kept overnight at 4-8°C. The rosette-forming cells in the pellet were gently resuspended and further purified by Ficoll-Hypaque gradient ccntrifugation. The AET-SRBC were lysed by a hypotonic ammonium chloride buffer solution, resulting in purified T cells.
Separation of ConA-SRBC rosetted T cells. Separation of human suppressor and helper-T cells by ConA-coated SRBC was performed by the methods described by Makonkawkeyoon, et al. (11). Briefly, purified T cells, 1 x 106/ml, were mixed with 0.1 ml of 1% ConA-SRBC. The mixture was incubated at 37°C, 5% CO2 for 30 min, centrifuged at 600 xgx2 min, and then incubated at 37°C for another 30 min. The ConA-SRBC rosetted T cells were separated from the nonrosetted T cells by Ficoll-Hypaquc gradient ccntrifugation. ConA-SRBC were lysed by an ammonium-chloride buffer solution. The ConA-SRBC rosetted and nonrosetted T cells were washed once with RPMI medium, and then adjusted to a concentration of 1 x 106 cells/ ml.
A 1% ConA-SRBC suspension was prepared using equal volumes of 2% SRBC in phosphate buffered saline (PBS), pH 7.4, mixed with 1:800 of ConA solution (1:800 ConA = 1 μg ConA/μl diluted with PBS, pH 7.4, to 1:800). The mixture was incubated for 1 hr at 37°C with frequent mixing and then washed twice with PBS. The ConA-SRBC suspension was diluted to 1% with RPMI medium.
Separation of CD8+ T cells. A T-cell suspension, 1 x 106 cells in 100 μl RPMI medium, was treated with 5 μg of anti-T4 monoclonal antibody (Ortho Pharmaceutical Corporation, Raritan, New Jersey, U.S.A.). The cell suspension was incubated at 4°C for 30 min, washed twice with RPMI medium, and complement (Low-Tox® rabbit complement; Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) was added at a final dilution of 1:10. The cells were incubated for an additional 30 min at 37°C, washed once with RPMI 1640 medium, and then centrifuged through Ficoll-Hypaque medium to remove effete and dead cells. The cell preparation was checked for CD8 + cells by indirect immunofluorescence using anti-T8 monoclonal antibody; it contained more than 92% CD8+ cells.
Culture conditions. Normal PBML were used as the indicator cells for measuring augmentation or suppression of the isolated PBML, T cells, and T-cell subpopulations. The normal indicator PBML, 1 x106/ml, in a 10 x75 mm plastic lube (Falcon) were mixed with different concentrations of isolated PBML, T cells, and T-cell subpopulations. A final dilution of 1:200 of poke-weed mitogen (PWM; GIBCO) was mixed in the cell culture and incubated at 37°C in 5% CO2 on a rocker platform (Belco) for 6 days for the determination of plaque-forming cells (PFC) (performed by reverse hemolytic plaque assay) and for 9 days for the determination of IgG concentrations (performed by sandwich ELISA).
Reverse hemolytic plaque assay. The determination of PFC was done using a reverse hemolytic plaque assay (13). Briefly, SRBC were coated with staphylococcal protein A (SPA; Pharmacia Fine Chemicals, Inc., Piscataway, New Jersey, U.S.A.) by chromic chloride (Baker Co., Phillipsburg, New Jersey, U.S.A.) as the coupling agent. The SPA-SRBC were mixed with cell-mixing culture and agarose (Accurate Chemical, Hicksville, New York, U.S.A.) and then poured onto an agarose-precoated petri dish. The plate was incubated at 37°C, 5% CO2 for 2 hr, and then overlaid with a 1:50 dilution of IgG fraction of rabbit anti-human IgG + M + A (Cappcl Laboratories, Down-ington, Pennsylvania, U.S.A.). After 2 hr of incubation, the rabbit antiserum was removed, fresh guinea pig complement diluted 1:40 was added, and the plate was incubated for 1 hr. The number of PFC was determined under a dissecting microscope; the data are expressed as PFC per 106 PBML.
In vitro immunoglobulin secretion. The amount of secreted IgG in the culture supernatant was determined by a sandwich ELISA (12). Briefly, 200 μl rabbit anti-human IgG (Cappel; dilution 1:2000) was coated onto each well. The wells were washed with PBS-Tween, and the culture supernatant, 200 μl in an appropriate dilution, was added. The plate was incubated and washed with PBS-Tween. Rabbit anti-human IgG peroxidase conjugate (200 μl) was added. The plate was incubated, washed, and then 200 μl of substrate was added. The reacting microtiter plate was read at 492 nm in a spectrophotometer (Microplate Auto-reader EL309; Bio-Tek Instruments, Wi-nooski, Vermont, U.S.A.). Concentrations of IgG were calculated from a standard curve obtained by using known concentrations of standard human IgG (Baxter Health Care Pharmaceuticals, Hyland Division, Glen-dale, California, U.S.A.).
Lymphocyte transformation test. PBML were plated in triplicate at 1 x 105 cells per well in a 96-well, flat-bottom plate in 200 μlof 10% FBS-RPMI medium. PHA-P (Difco Laboratories, Detroit, Michigan, U.S.A.) 0.2 μg/well was added, the culture was incubated for 72 hr and 0.2 μCi [3H]-thymidine (Amersham, UK) in 50 μl RPMI medium was added, and the culture was incubated for another 18 hr. The cells in tissue culture wells were collected by a cell harvester (PHD; Cambridge Technology, Inc.), and [3H]-thymidine uptake was determined by a liquid scintillation counter (LS 3801; Beckman Instruments, Inc., Ful-lcrton, California, U.S.A.).
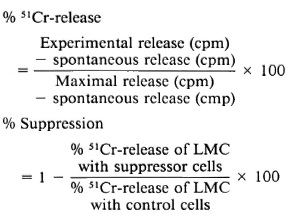
Lymphocyte-mediated cytotoxicity assay. A lymphocyte-mediated cytotoxicity (LMC) assay was performed in triplicate in screw-cap tubes. Each culture tube contained 3.0 x 106 of 51Cr-chicken red blood cells, 1 x 106 PBML, and 5 μl PHA-P (Difco). When this technique was used for measuring augmentation or suppression, different quantities of cells were added to this assay system. After incubation at 37°C, 5% CO2 for 48 hr and gentle shaking, the solution was centrifuged at 400 x g x 10 min, and 0.75 ml supernatant fluid was measured for the amount of 51Cr released by a gamma counter (Searle Analytic, Inc., Skokie, Illinois, U.S.A.). Calculations were done as follows:
RESULTS
Figure 1 shows lymphocyte transformation in the PBML of 17 lepromatous (LL), 9 borderline lepromatous (BL), 9 borderline (BB), 23 borderline tuberculoid (BT), and 8 tuberculoid (TT) leprosy patients and 20 normal controls when stimulated with PHA-P. Although there are no statistically significant differences among the various groups of leprosy patients when compared with normal controls, it is noted that the lymphocyte transformation test (LTT) activities of normal controls and TT leprosy patients arc not as widely distributed as seen in BT, BB, BL, and LL leprosy patients. Figure 2 shows the lymphocyte-mediated cytotoxicity (LMC) of PBML in 21 LL, 9 BL, 8 BB, 18 BT and 6 TT patients and 19 normal controls when stimulated with PHA-P. The pattern of LMC activities among the various leprosy patient groups and normal controls is very similar to the LTT activities. Figure 3 depicts the numbers of PFC in 19 LL, 8 BL, 9 BB, 21 BT, and 11 TT patients and 46 normal controls. The pattern of PFC distribution is quite similar to the LTT and LMC activities. Figure 4 illustrates the suppressive activities of ConA-stimulated PBML of BL/LL and TT patients and normal controls by the LMC assay. Nine BL/LL patients with a bacterial index (BI) of 3 or more (average 3.92) had significantly less suppression than 16 normal controls (p < 0.05). However, 6 BL/ LL patients with a BI of less than 3 (average 0.95) showed no significantly lower suppression than 16 normal controls (p > 0.05). Ten TT patients had no significantly lower suppression than 16 normal controls (p > 0.05). Figure 5 shows the suppressive activity of CD8 + T cells from 6 LL patients and 6 normal controls using the PFC response as the assay system. The suppressive activity of CD8 + T cells from 6 LL patients is significantly less than that from normal controls at 1%, 10% and 80% of CD8+ T cells added (p < 0.001, p < 0.001 and p < 0.001, respectively). Figure 5 also shows the suppressive activity of ConA-SRBC rosetted T cells from 16 LL patients and 16 normal controls using an ELISA for measuring IgG production. The suppressive activity of ConA-SRBC rosetted T cells from 16 LL patients was significantly lower than that from normal controls at 5%, 10%, 20%, 40% and 80% (p < 0.005, p < 0.01, p < 0.001, p < 0.001 and p < 0.005, respectively).
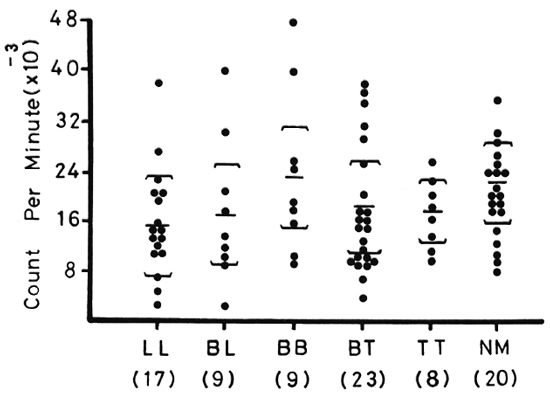
Fig. 1. Lymphocyte transformation test (LTT) in peripheral blood mononuclear leukocytes (PBML) of various groups of leprosy patients and normal controls when stimulated with PHA-P; EE = mean ± S.D.
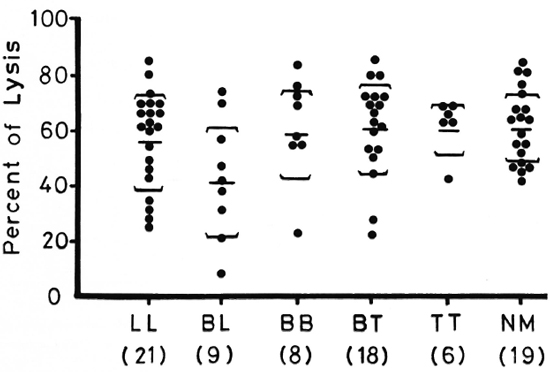
Fig. 2. Lymphocyte-mediated cytotoxicity (LMC) in PBML of various groups of leprosy patients and normal controls when stimulated with PHA-P; EE = mean ± S.D.
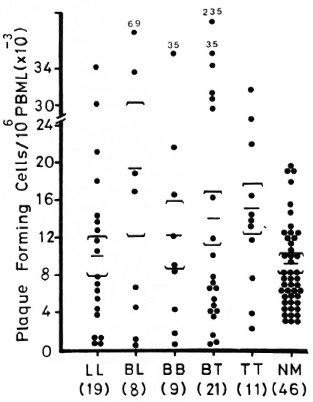
Fig. 3. Plaque-forming cells (PFC) in PBML of various groups of leprosy patients and normal controls when stimulated with pokeweed mitogen (PWM); EE = mean ± S.D.
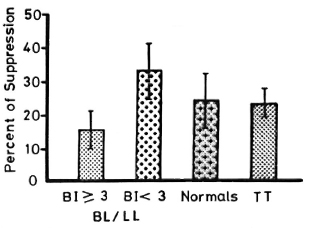
Fig. 4. Suppressive activities of ConA-stimulated PBML of BL/LL leprosy patients with a bacterial index (BI) > 3 and a BI < 3 and TT leprosy patients compared to normal controls using the LMC assay; ft = mean ± S.D.
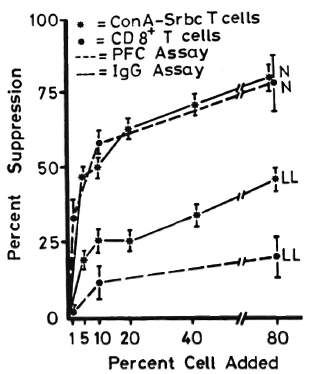
Fig. 5. Suppressive activities of CDS + T cells and ConA-SRBC rosetted T cells from LL leprosy patients and normal controls using PFC and production of IgG as the measuring systems; error bars = mean ± S.D.
DISCUSSION
Early reviews of the defects in CMI of lepromatous leprosy cited considerable data indicating a generalized defect in CMI. These data were derived from skin sensitization tests to dinitrochlorobenzenc (DNCB) and picryl chloride and in vitro proliferation assays of patient lymphocytes to various antigens and mitogens (5,21). It was noted that while the inability of lepromatous patients to respond to M. leprae was profound, the generalized immunologic ancrgy associated with several forms of leprosy is comparatively weak. Moreover, lepromatous patients do not seem highly susceptible to other infections, favoring the view that lepromatous patients are specifically anergic to M. leprae (5). Recent reports continue to agree with this view by stressing that the lepromatous patient will give a positive PPD skin test because tuberculin and lepromin arc crossrcactive (9).
We have reinvestigated the CMI response of PBML from various types of untreated leprosy patients using LTT and LMC assays stimulated with mitogens, PHA-P or ConA. The plasma inhibitory factors in leprosy patients were also investigated using 10% and 50% homologous, heterologous and AB plasma in the tissue culture system. Although the mean ± S.D. of the LTT from normal controls was not significantly different from the mean ± S.D. of the LL, BL, BB, BT and TT leprosy patients when stimulated with PHA-P, it was noted that the counts per minute (cpm) of normal controls and TT patients were less variable. Similar results were also obtained when stimulated with ConA (data not shown). Similar distributions of cpm in the LTT were obtained when 10% or 50% of plasma from normal volunteers or AB blood groups or leprosy patients were incorporated into the tissue culture system (data not shown). The same patterns of distribution of the percentage of lysis from the LMC assay in various types of leprosy patients were also obtained in this study. This finding suggests the variability of immunoregulation in BT, BB, BL and LL patients. It may reflect the wide variability of T-helper and T-suppressor cell ratios. The ratio of T-helper and T-suppressor cells in the circulation of lepromatous leprosy patients varies greatly and correlates well with their bacterial index (26). When the immunoregulation for humoral-mediated immunity (HMI) in various types of leprosy was evaluated using the reverse hemolytic plaque assay measuring PFC, there were also no statistically significant differences between normal controls and the various types of leprosy. Wide variations were observed in BT, BB, BL and LL patients.
The immunoregulatory role of PBML and T-cell subpopulations from various types of leprosy patients was evaluated in CMI and HMI responses using the LMC and reverse hemolytic plaque assays measuring PFC and ELISA quantitation of IgG. There was significantly lower suppressive activity of ConA-stimulated PBML from BL/LL patients with a BI > 3 (average 3.92) than from BL/LL patients with a BI < 3 (average 0.95) when compared with normal controls by the LMC assay. When ConA-stimulated PBML from TT patients was compared with normal controls in the regulation of LMC, there was no significant difference. This test showed that there was an immune aberration of suppressive activity in the ConA-stimulatcd PBML of BL/LL patients having a BI > 3 and the suppressive activity was normal when the BI was < 3. The suppressive activity of ConA-stimulated PBML in BL/LL patients was further evaluated by the isolation and purification of T-ccll subpopulations from ConA-stimulated PBML of BL/LL patients and then mixed with normal PBML and PWM as indicator cells for measuring immunoregulation of the T-cell subpopulations. The CD8 + T cells from BL/ LL patients showed highly significant differences of lower suppression than did the CD8+ T cells from normal controls in the assay measuring PFC. Similar results were obtained when ConA-SRBC T cells from BL/LL patients were compared with normal controls when assayed by an ELISA measuring IgG concentrations. We had demonstrated that ConA-SRBC rosetted T cells from normal subjects have highly suppressive activity in immune-regulation (11). The ConA-SRBC rosetted T-cell subpopulations from BL/LL patients also showed an aberration of immunosuppression similar to the CD8+ T cells.
Although it was suggested that the functional impairment of ConA-induced suppressor cells in lepromatous leprosy could be due to an intrinsic defect in one or many of the components of the suppressor circuit, it was also suggested that there is a link between the reduction of Leu-8+ cells and the low functional suppressor response of the PBML from LL patients after M. leprae stimulation. The low frequency of Leu-8 + cells in LL patients was a clue to the inability of PBML from these patients to generate specific and nonspecific suppression of T-cell proliferation and may be related to the inability of LL patients to resist M. leprae (22). The significance of our findings of the immune aberration of suppressor-T cells in lepromatous leprosy has to be further evaluated and examined more thoroughly to increase our understanding of the abnormality of CMI in leprosy patients.
Acknowledgment. This research was supported by USAID/PSTC Program Grant no. 936-5542-G-00-5056-00.
REFERENCES
1. Bjune, G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohacmagglutinin. Clin. Exp. Immunol. 36(1979)479-487.
2. Bloom, B. R. and Godal, T. Selective primary health care: strategies for the control of disease in the developing world. V. Leprosy. Rev. Infect. Dis. 5(1983)765-780.
3. Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21 Suppl. 97(1968)77-89.
4. Bullock, W. E. Studies of immune mechanisms in leprosy. I. Depression of delayed allergic responses to skin test antigens. N. Engl. J. Med. 278(1968)298-304.
5. Bullock, W. E. Immunobiology of leprosy. In: Immunology of Human Infection, Part I. Nah-mias, A. J. and O'Reilly, R. J. , eds. New York: Plenum, 1981, pp. 369-390.
6. Bullock, W. E. , Watson, S. , Nelson, K. E. , Schauf, V. , Makonkawkeyoon, S. and Jacouson, R. R. Aberrant immunorcgulatory control of B lymphocyte function in lepromatous leprosy. Clin. Exp. Immunol. 49(1982)105-114.
7. Haregewoin, A. , Godal. T. , Mustafa, A. S. , Be-lehu, A. and Yemaneberhan, T. T cell conditioned media reverse T cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
8. Hunt, S. V. Preparative immunosclection of lymphocyte populations. In: Handbook of Experimental Immunology, Vol. 2. Weir, D. M. , ed. Oxford: Blackwell Scientific Publications, 1986, chapter 55, pp. 55. 1-55. 18.
9. Kaplan, G. and Cohn, Z. A. The immunobiology of leprosy. Int. Rev. Exp. Pathol. 28(1986)45-78.
10. Kaplan, G. , Weinstein, D. E. , Steinman, R. M. , Levis, W. R. , Elvers, U. , Patarroyo, M. E. and Cohn, Z. A. An analysis of in vitro T cell responsiveness in lepromatous leprosy. J. Exp. Med. 162(1985)917-929.
11. Makonkawkeyoon, S. and Kasinrerk, W. Separation of human suppressor and helper T cells by concanavalin A-coatcd sheep erythrocytes. Asian Pac. J. Allergy Immunol, (in press).
12. Makonkawkeyoon. S. , Kasinrerk, W. and Vithayasai, V. Regulation of immunoglobulin secretion by T lymphocytes in human malaria. Asian Pac. J. Allergy Immunol. 4(1986)13-17.
13. Makonkawkeyoon, S. and Vithayasai. V. A pre-amplified reverse hemolytic plaque assay. J. Immunol. Methods 62(1983)365-371.
14. Mehra. V. , Brennan, P. J. , Rada, E. , Convit, J. and Bloom, B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308(1984)194-196.
15. Mehra, V. , Mason, L. H. , Fields, J. P. and Bloom, B. R. Lepromin-induced suppressor cells in patients with leprosy. J. Immunol. 123(1979)1813-1817.
16. Mehra. V. . Mason, L. H. , Rothman, W. , Reinherz, E. , Schlossman, S. F. and Bloom, B. R. Delineation of a human T cell subset responsible for lepromin-induced suppression in leprosy patients. J. Immunol. 125(1980)1183-1188.
17. Mendes, E. . Raphael, A. , Mota, N. G. S. and Mendes, N. F. Cell-mediated immunity in leprosy and transfer of delayed hypersensitivity reactions. J. Allergy Clin. Immunol. 53(1974)223-229.
18. Myrvang, B. . Godal, T. , Ridley, D. S. , Fro-land, S. S. and Song, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
19. Nath. I. and Singh, R. The suppressive effect of M. leprae on the in vitro proliferative responses of lymphocytes from patients with leprosy. Clin. Exp. Immunol. 41(1980)406-414.
20. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
21. Sansonetti, P. and Lagrange, P. H. The immunology of leprosy: speculations on the leprosy spectrum. Rev. Infect. Dis. 3(1981)422-469.
22. Sasiain, M. del C. de la Barrera, S. , Valdez, R. and Balina, L. M. Reduced suppressor cell response to Mycobacterium leprae in lepromatous leprosy. Infect. Immun. 57(1989)951-956.
23. Stoner, G. L. , Mshana, R. N. , Touw, T. and Belehu, A. Studies on the defect in cell-mediated immunity in lepromatous leprosy using HLA-D-identical siblings. Absence of circulating suppressor cells and evidence that the defect is in the T-lymphocyte, rather than the monocyte, population. Scand. J. Immunol. 15(1982)33-48.
24. Turk, J. L. and Bryceson, A. D. M. Immunological phenomena in leprosy and related diseases. Adv. Immunol. 13(1971)209-266.
25. Waldorf, D. S. , Sheagren, J. N. , Trautman, J. R. and Block, J. B. Impaired delayed hypersensitivity in patients with lepromatous leprosy. Lancet 2(1966)773-776.
26. Wallach, D. , Cottenot, F. and Bach, M. -A. Imbalances in T cell subpopulations in lepromatous leprosy. Int. J. Lepr. 50(1982)282-290.
1. Ph.D.; Department of Clinical Immunology, Faculty of Associated Medical Sciences; Chiang Mai University, Chiang Mai 50002, Thailand.
2. M.S.; Department of Clinical Immunology, Faculty of Associated Medical Sciences; Chiang Mai University, Chiang Mai 50002, Thailand.
3. M.S.. Department of Clinical Immunology, Faculty of Associated Medical Sciences; Chiang Mai University, Chiang Mai 50002, Thailand.
4. M.D., Ph.D., Department of Microbiology, Faculty of Medicine. Chiang Mai University, Chiang Mai 50002, Thailand.
Received for publication on 30 May 1989.
Accepted for publication in revised form on 8 November 1989.