- Volume 58 , Number 2
- Page: 311–8
Immunologic defects in leprosy patients. II. Interleukin 1, Interleukin 2, and Interferon production in leprosy patients
ABSTRACT
The capabilities of monocytes and lymphocytes in peripheral blood mononuclear leukocytes (PBML) to produce interleukin-1 (IL-1), IL-2, and interferon (IFN), respectively, were evaluated in various types and treatments of leprosy patients. IL-1 production in response to lipopolysaccharide was significantly lower in LL, BL, BB, and BT patients than in normal controls. However, there were no differences in IL-1 levels between TT patients and normal controls. The percentages of nonspecific-esterase-positive cells adhering to the plastic surfaces were not different in LL, BB and TT patients when compared to normal controls. However, they were significantly higher in BT and BL patients than in normal controls.When PBML f rom leprosy patients were stimulated with concanavalin-A (ConA) for IL-2 production, there were no differences in the IL-2 levels in treated BL/LL, untreated BL/LL, treated BT/TT, and untreated BT/TT patients compared to normal controls. Similar results were obtained when PBML were stimulated with phytohemag-glutinin-P (PHA-P). However, when purified protein derivative (PPD) was used as the stimulating agent, there were significantly lower IL-2 levels in treated BL/LL, untreated BL/LL, treated BT/TT, and untreated BT/TT patients when compared to normal controls. There were also lower IL-2 levels in untreated BL/LL and BT/TT patients compared to treated BL/LL and BT/ TT patients, respectively.
PBML were stimulated with PHA-P or ConA for IFN production. There were no differences in the IFN levels in treated BL/ LL, untreated BL/LL, treated BT/TT, and untreated BT/TT patients compared to normal controls. However, when PPD was used as the stimulating agent, there were lower IFN levels in treated BL/LL, untreated BL/ LL, and treated BT/TT patients compared to normal controls.
RÉSUMÉ
On a évalué dans différents types de lèpre, et au cours de différents schémas de traitement, la capacité des monocytes et des lymphocytes du sang périphérique, à produire de I'interleukine-1 (IL-1), I'interleukine-2, et de l'interferon (IFN). La production d'interleukine-1, en réponse au lipopolysaccharides, était significati-vement abaissée chez les malades atteints de lèpre LL, BL, BB, et BT, par rapport aux témoins normaux. Néanmoins, on n'a pu noter aucune différence dans les taux d'interleukine-1 entre les malades TT et les témoins en bonne santé. Les pourcentages de cellules positives pour l'esterase, mais non spécifiques, qui adhèrent aux surfaces plastiques, n'étaient pas différents chez les malades LL, BB et TT lorsqu'on les comparait à des témoins normaux. Néanmoins, les pourcentages était significantivément élevée dans de lèpre BT et BL par rapport aux témoins normaux.Lorsque ces leucocytes mononucléaires du sang périphérique, recueillis chez des malades de la lèpre, étaient stimulés par la concanavaline-A (ConA), en vue de produire de l'interleukine-2, aucune difference n'a été notée dans les taux d'inlerleukine-2 chez les malades BL/LL traités, chez les malades BL/LL non traités, chez les malades BT/TT traités, et chez les sujets BT/TT non traités, par rapport aux témoins normaux. Dos résultats semblables ont été obtenus lorsque ces leucocytes mononucléaires étaient stimulés par la phy-tohémagglutinine-P(PHA-P). Toutefois, lorsqu'on utilisait le dérivé protéinique purifié (PPD) comme agent de stimulation, on a relevé des taux d'interleukine-2 significativement plus faibles chez les BL/LL traités, les BL/LL non traités, les BT/TT traités, et les BT/TT non traités, par comparaison avec les témoins normaux. On a également observé un abaissement des taux d'interleukine-2 chez, les sujets BL/LL non traités, et chez les malades BT/TT non traités, lorsqu'on les comparait aux individus BL/LL traités et aux BT/TT traités.
La production d'interferon par les leucocytes mononucléaires du sang périphérique a été stimulée par le PHA-P ou la ConA.
Aucune différence n'a été notée dans les taux d'interferon chez les malades BL/LL traités, les BL/LL non traités, les BT/TT traités, et les BT/TT non traités, par rapport aux témoins normaux. Cependant, lorsque le PPD était utilisé comme agent de stimulation, on a enregistré des taux d'interferon plus faibles chez les BL/LL traités, BL/LL non traités, et BT/TT traités, que chez les témoins normaux.
RESUMEN
Se evaluó la capacidad de los monocitos y los lin-focitos de la sangre periférica de pacientes con varios tipos de lepra y tratamientos para producir, respectivamente, interleucina-1 (IL-1) o IL-2 c interferón (IFN). La producción de IL-1 en respuesta a lipopolisacárido fue significativamente mayor en los controles sanos que en los pacientes LL, BL, BB y BT. Los pacientes TT y los controles sanos produjeron niveles equivalentes de IL-1. Los porcentajes de células adherentes esterasa-positivas fueron similares en los pacientes LL, BB y TT y en los controles sanos. Sin embargo, ellos fueron significativemente mayor en los pacientes BT y BL que en los controles normales.La producción de IL-2 estimulada por concanava-lina A (Con-A) o por fitohemaglutinina P (FHA-P) fue comparable en los pacientes BL/LL tratados y no tratados, en los BT/TT tratados y no tratados y en los controles sanos. Sin embargo, cuando la estimulación de los leucocitos se hizo con PPD, los pacientes BL/ LL tratados y no tratados y los BT/TT tratados y no tratados, tuvieron niveles de IL-2 significativamente menores que los controles normales. Los pacientes BL/ LL y BT/TT no tratados produjeron menos IL-2 que los correspondientes pacientes tratados.
La producción de IFN, estimulada con FHA-P o Con-A, fue similar en los pacientes BL/LL tratados y no tratados, en los BT/TT tratados y no tratados, y en los controles sanos. Con el PPD, sin embargo, los niveles de IFN en los pacientes BL/LL y BT/TT, tratados y no tratados, fueron más bajos que en los controles normales.
Many hypotheses have attempted to explain the cell-mediated immunity (CMI) defect in lepromatous leprosy. However, the immunologic defect(s) leading to the reduced or absent cellular immunity to Mycobacterium leprae in lepromatous patients is not as yet fully understood. Immune responses arc controlled, in part, by soluble mediators which are produced by cells of the lymphoreticular system. Some of these soluble mediators, for example, interleukin 1 (IL-1) and interleukin 2 (IL-2) and interferon gamma (IFN-γ), exert various im-munoregulatory effects on the immune system. Recent evidence points to a lack of lymphokinc production in cells from lepromatous leprosy patients (3,5,8,11,13).
IL-1, a monokine, exerts a variety of im-munoregulatory effects, among which is an ability to react with T cells, preparing them to respond to subsequent signals. Whether IL-1, which is necessary along with properly presented antigen for T-cell stimulation, is properly secreted by the monocytes of patients with lepromatous leprosy is also in question. Some lepromatous patients' production of IL-1 in response to lipopolysac-charide is poor (17). However, other reports show that production of IL-1 in response to M. leprae is as good as that of BCG (4).
IL-2 plays a major role in the immune response to infectious agents by activating the immune responses of the host. Defective IL-2 production has been reported in many immunodeficiency diseases. Maximal IL-2 synthesis and secretion require both antigen (or mitogen) and IL-1 (10). Recently, it has been shown that IL-2 treatment in mice resulted in limiting mycobacterial infections (6). It was also shown that T-cell-condi-tioned medium, purified IL-2. or recombinant IL-2, when given with M. leprae, stimulated T-cell proliferation in lepromatous leprosy patients' cells (3,4). However, others found only sporadic positive effects with IL-2 (8,13,15).
Macrophages express nonspecific bactericidal activity which is greatly enhanced by local production of immune interferon (IFN-γ) by antigen-reactive T cells (12). This major macrophage-activating factor, IFN-7, was found in antigen- and mitogen-stimu-lated lymphocyte culture supernatants (12). IFN-7 has been shown to enhance oxidative metabolism in cultured human monocytes and to render them capable of killing intracellular parasites (7). Peripheral blood mononuclear leukocytes (PBML) of lepromatous patients were defective in IFN-7 production, but some patients produced interferon when they were simultaneously given M. leprae and IL-2 IFN-γ appears to be the principal factor secreted by antigen-stimulated lymphocytes that activates macrophages, in the sense that the phagocytes more efficiently inhibit or kill pathogenic microorganisms. Deficient macrophage activation may be a characteristic of borderline lepromatous and polar lepromatous leprosy (12).
We attempted to investigate the defect of immunoregulation in CMI by studying the ability of monocytes to produce IL-1 and PBML to produce IL-2 and IFN using strong nonspecific stimulants, PHA-P and ConA, and a weak specific stimulant, PPD, in various types of leprosy patients and normal controls.
MATERIALS AND METHODS
Leprosy patients and normal controls. All treated and untreated leprosy patients were seen at the McKean Rehabilitation Center or the Chiang Mai Christian skin clinic in Chiang Mai, Thailand. All patients were diagnosed and classified by the Ridley and Jopling classification based on clinical and histopathological findings (16). Untreated leprosy patients were defined as new cases or those treated with dapsone (DDS) for not more than 6 weeks. Treated patients were cases treated with antileprosy drugs for more than 6 weeks. Normal controls were volunteer laboratory personnel and students (all PPD skin-test positive).
Preparation of PBML. Heparinized venous blood was obtained from normal controls and leprosy patients. PBML were prepared by the standard Ficoll-Hypaque density centrifugation (2) and washed three times with RPMI 1640 medium (GLBCO, Grand Island, New York, U.S.A.). The concentration of the PBML was adjusted to 1 x 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS; GIBCO). The RPMI 1640 medium always contained 100 units of penicillin and 100 μg of streptomycin per ml.
Separation of monocytes. PBML (4 x 106 cells in 2.0 ml medium) were added onto two 35 x 10 mm plastic plates (Nunc, Kam-strup, Denmark), and the plates were incubated at 37°C, 5% CO2 for 2-4 hr. Nonadherent cells were washed off by washing both plates three times with 10% FBS-RPMI 1640 medium, and the number of cells from one of the plates was counted. The PBML, nonadherent and adherent cells, were stained with nonspecific esterase (NSE) stain. The actual number of monocytes adhering on the plate was calculated by subtracting the number of cells washed off from the number of PBML added, and the percentage of NSE-positive cells in the adherent cells on the plate was determined and corrected. The desired concentration of NSE-positive cells or monocytes could then be achieved by adding a certain amount of 10% FBS-RPMI 1640 medium.
Production and determination of IL-1. The adhered monocytes of normal controls or leprosy patients on plastic plates were adjusted to 2 x 105 NSE-positive cells/ml by adding various calculated amounts of 10% FBS-RPMI 1640 medium. Lipopoly-saccharide (LPS; Escherichia coli 0127:B8; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) 20 μg/ml was added and incubated at 37°C, 5% CO2 for 24 hr. Cell suspension was collected, centrifuged, and the super-natants kept at -70°C for the IL-1 assay.
Determination of IL-1 was done with a thymocyte suspension from 8-wcck-old inbred BALB/c mice. The thymocyte suspension was prepared by gently homogenizing the thymus gland in a sterile mesh with a sterile rubber plunger, washing twice with RPMI 1640 medium, and adjusting to 3 x 107 cells/ml. Samples for the IL-1 assay were diluted in serial twofold dilutions with a final volume of 600 μl for each dilution. The thymocyte suspension, 100 μl, was added and mixed. Then 30 μl of PHA-P (Difco Laboratories, Detroit, Michigan, U.S.A.; 20 μg/ml) was added and mixed well. After 200 μl of the mixture was added in triplicate to wells of 96-well, flat-bottom tissue-culture plates (Nunc) and incubated at 37°C, 5% CO2 for 72 hr, 25 μl of tritiated thymidine (8 μCi/ml) was added to each well, and the plates were incubated for 2 hr. The culture was harvested onto glass microfiber filters using a cell harvester (PHD; Cambridge Technology, Inc.), and the radioactivity was counted in a liquid scintillation counter (LS 3801; Beckman Scientific Instruments, Inc., Fullerton, California, U.S.A.). Levels of IL-1 were expressed in unit per milliliter by comparison with commercial standard human IL-1 (Human in-terleukin 1, ultrapure; Genzyme, Massachusetts, U.S.A.).
Production and determination of IL-2. PBML (1 x 106 cells/ml) were stimulated with 2 μg/ml PHA-P (Wellcome, U.K.) or 40 μg/ml ConA (Sigma) or 10 μg/ml PPD (Connaught, Canada) and incubated at 37°C, 5% CO2 for 18 hr for mitogens and 48 hr for PPD. All of these optimal conditions were determined previously by our own laboratory. The cell cultures were harvested and centrifuged. To the supernatant was added 10 mg/ml α-methyl-D-mannoside (Sigma). The supernatant was sterile filtered and then stored at - 70°C for the IL-2 assay.
Samples for the IL-2 assay were diluted in serial twofold dilutions of 100 jul in triplicate and added to each well of a 96-well tissue-culture plate. The IL-2-dependent mouse T-cell line, CTLL-2, 100 μ1(8 x 103 cells/100 μl), was added to each well and the plate was incubated for 24 hr. After 50 μl of 3H-thymidine (0.2 μCi/50 μl) was added for 18 hr, the cultures were harvested onto glass microfiber filters and the 3H-thymidine incorporation was determined by a liquid scintillation counter (Beckman; LS 3801). The levels of IL-2 were expressed in units per milliliter by comparison with the commercial standard human IL-2.
Production and determination of interferon. The production of interferon was similar to that of IL-2. Samples for the interferon assay were diluted in serial twofold dilutions as in the IL-2 assay. FL 5-1 cells, 75 μl (1.5 x 105 cells/ml), were then added to each well. After 24 hr of incubation, 50 μl of vesicular stomatitis virus (2 x 103 plaque-forming units) was inoculated into each well. The cytopathogenic effect (CPE) was examined after a further 24-hr incubation. Complete or 100% CPE was obtained in the virus control well, while the cell control well of FL 5-1 was completely intact. Cells in each well were fixed with 10% Formalin and then stained with 0.4% crystal violet in 70% methanol. Antiviral activity was expressed in interferon titer units and is defined as the reciprocal of the highest dilution of the sample which reduced the number of viral plaques by 50%. In our IFN assay, reference laboratory standard IFN was produced by PHA-P (2 μg/ ml) stimulation of 2 x 106 cells/ml human PBML for 48 hr.
RESULTS
The Table shows the separation and purification of monocytes from the PBML of leprosy patients and normal controls. The percentages of adhering cells in BT, BB, BL, and LL patients are significantly lower than in normal controls (p < 0.001, p < 0.005, p < 0.001, and p < 0.005, respectively). However, there is no significant difference between TT patients and normal controls. When the percentages of the adhering NSE-positivc cells in TT, BB and LL patients are compared with normal controls there arc no significant differences (all have p > 0.05). However, significant higher values were obtained in BT and BL patients than in normal controls (p < 0.05 and p < 0.01, respectively).
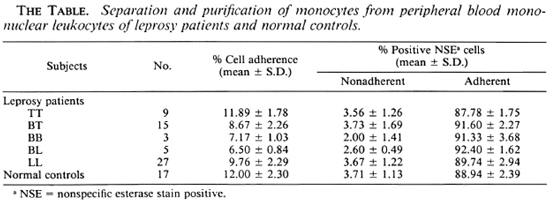
Figure 1 depicts IL-1 production from leprosy patients and normal controls stimulated with lipopolysaccharide (LPS). There are significantly lower IL-1 levels in LL, BL, BB and BT patients compared to normal controls (p < 0.001, p < 0.05, p < 0.005, and p < 0.05, respectively). However, there is no significant difference between TT patients and normal controls.
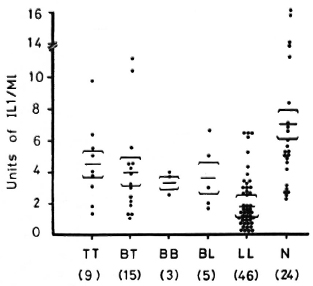
Fig. 1. Interleukin 1 (IL-1) production from 2 x105/ml monocytes of LL. BL, BB, BT and TT leprosy patients and normal controls when stimulated with lipopolysaccharidc (LPS).  = mean ± S.E.; figures in parentheses = number of subjects. NOTE: a) Unstimulated monocyte cultures from LL, BL, BB, BT and TT patients and normal controls contained IL-1 activities in cpm, mean ± S.E. (range) = 2630 ± 384 (1233-4214), 2484 ± 270 (2017-3518), 3030 + 275 (2346-3694), 3194 ± 619 (1836-561 1), and 4011 ± 552 (2177-5461), and 4040 ± 499 (2533-6147), respectively, b) LPS-stimulated monocyte cultures from LL, BL. BB, BT and TT patients and normal controls contained IL-1 activities in cpm, mean ± S.E. (range) = 5196 ± 744 (3146-9234), 5929 ± 443 (4850-7193), 7356 ± 424(6167-8097),6951 ± 1206(3286-12,387), and 9151 ± 960 (5604-12,190), and 9807 ± 1262 (6438-1 5,817), respectively, c) Thymocyte only, mean ± S.E. (range) = 226 ± 88 ( 103-347) cpm. Thymocyte + PHA-P, mean ± S.E. (range) = 634 ± 73 (525-732) cpm.
= mean ± S.E.; figures in parentheses = number of subjects. NOTE: a) Unstimulated monocyte cultures from LL, BL, BB, BT and TT patients and normal controls contained IL-1 activities in cpm, mean ± S.E. (range) = 2630 ± 384 (1233-4214), 2484 ± 270 (2017-3518), 3030 + 275 (2346-3694), 3194 ± 619 (1836-561 1), and 4011 ± 552 (2177-5461), and 4040 ± 499 (2533-6147), respectively, b) LPS-stimulated monocyte cultures from LL, BL. BB, BT and TT patients and normal controls contained IL-1 activities in cpm, mean ± S.E. (range) = 5196 ± 744 (3146-9234), 5929 ± 443 (4850-7193), 7356 ± 424(6167-8097),6951 ± 1206(3286-12,387), and 9151 ± 960 (5604-12,190), and 9807 ± 1262 (6438-1 5,817), respectively, c) Thymocyte only, mean ± S.E. (range) = 226 ± 88 ( 103-347) cpm. Thymocyte + PHA-P, mean ± S.E. (range) = 634 ± 73 (525-732) cpm.
Figure 2 illustrates IL-2 production from PBML of patients and normal controls when stimulated with ConA. IL-2 levels from these different groups of leprosy patients are not significantly different from normal controls.
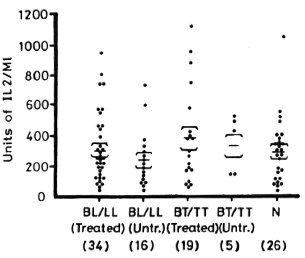
Fig. 2. IL-2 production from 1 x106/ml PBML of treated and untreated BL/LL and BT/TT patients and normal controls when stimulated with ConA.  = mean ± S.E.; figures in parentheses = number of subjects.
= mean ± S.E.; figures in parentheses = number of subjects.
Figure 3 shows the levels of IL-2 production from PBML of leprosy patients and normal controls when stimulated with PHA-P. IL-2 levels from the different groups of leprosy patients are also not different from the levels of normal controls.
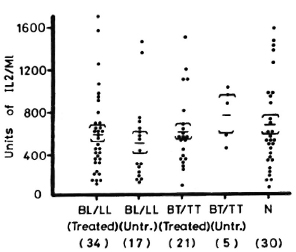
Fig. 3. IL-2 production from 1 x 106/ml PBML of treated and untreated BL/LL and BT/TT patients and normal controls when stimulated with PHA-P.  = mean ± S.E.; figures in parentheses = number of subjects.
= mean ± S.E.; figures in parentheses = number of subjects.
Figure 4 depicts the levels of IL-2 production from patients and normal controls when PBML are stimulated with PPD. The IL-2 levels from the treated BL/LL and treated BT/TT patients are significantly lower than normal controls (p < 0.01 and p < 0.01, respectively). IL-2 levels from untreated BL/LL and untreated BT/TT patients are highly significantly lower than in normal controls (p < 0.001 and p < 0.001, respectively). When treated BL/LL and treated BT/TT patients are compared with untreated BL/LL and untreated BT/TT patients, respectively, there arc significant differences (p < 0.05 and p < 0.05, respectively).
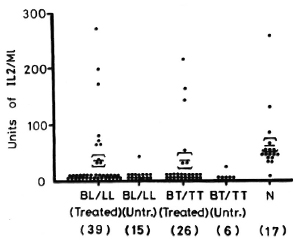
Fig. 4. IL-2 production from 1 x 106/ml PBML of treated and untreated BL/LL and BT/TT patients and normal controls when stimulated with PPD.  = mean ± S.E.; figures in parentheses = number of subjects.
= mean ± S.E.; figures in parentheses = number of subjects.
Figure 5 shows IFN production from leprosy patients and normal controls when stimulated with PHA. The IFN levels from the different groups of leprosy patients are not significantly different from normal controls.
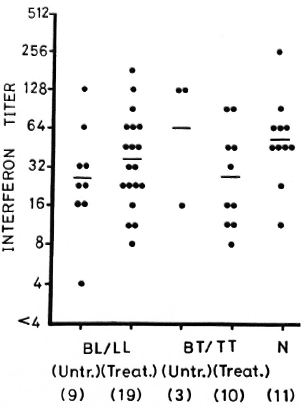
Fig. 5. Interferon (IFN) production from 1 x 106/ ml PBML of treated and untreated BL/LL and BT/TT patients and normal controls when stimulated with PHA-P. - = mean; figures in parentheses = number of subjects.
Figure 6 shows the IFN production from leprosy patients and normal controls when stimulated with PPD. IFN levels from treated BL/LL, untreated BL/LL and treated BT/TT patients arc significantly lower than those of the normal controls (p < 0.05, p < 0.01, and p < 0.01, respectively). However, IFN levels in the three untreated BT/TT patients do not show any difference from the normal controls.
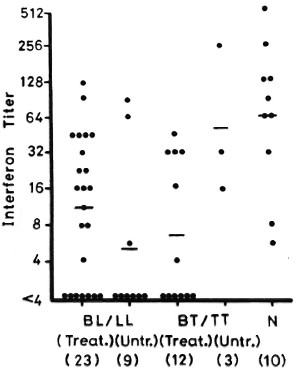
Fig. 6. IFN production from 1 x 106/ml PBML of treated and untreated BL/LL and BT/TT patients and normal controls when stimulated with PPD. - = mean; figures in parentheses = number of subjects.
DISCUSSION
Harcgewoin, et al. (3,4) presented data that T-cell-conditioned media, purified IL-2 or recombinant IL-2, when given with M. leprae, stimulated T-cell proliferation in lep-romatous patients' cells. However, the response was somewhat variable and suggested that some M. leprae-reactive T cells were present in lepromatous patients but were suppressed. Others found sporadic positive effects with IL-2 (13,15). Kaplan, et al. (8) reassessed M. leprae T cells proliferative responses in a large group of patients. They concluded that lepromatous patients were cither entirely anergic or very weakly positive and that IL-2 enhanced only weakly positive responses and then only by a small amount. In no case could reversal of anergy be shown. We have studied large numbers of treated BL/LL. untreated BL/LL, treated BT/TT and untreated BT/TT patients, along with considerable numbers of normal subjects, for IL-2 production stimulated with PHA-P or ConA or PPD or M. leprae. Our results show that IL-2 production from the various groups of leprosy patients mentioned above is not different from normal subjects when stimulated with cither PHA-P or ConA. However, when PPD is used as the stimulating agent, significantly lower IL-2 levels were found in treated and untreated BL/LL and treated and untreated BT/TT patients as compared to normal controls, and to untreated BL/LL as compared to treated BL/LL patients, and untreated BT/TT as compared to treated BT/ TT patients.
Our finding shows that IL-2 production is not different between normal controls and leprosy patients when stimulated by mitogens, such as PHA-P or ConA, which are strong stimulating agents. However, when stimulated by PPD, one can see differences in IL-2 production. We tried to stimulate IL-2 production in various types of leprosy patients and PPD skin-test-positive normal controls by M. leprae. However, very low levels or no IL-2 were produced by these subjects (9).IL-1 is a potent inducer of the production of lymphokines and cytokines by many different cells (1). Therefore, in theory, the inability to produce IL-1 could result in immunologic defects. To date, however, no specific disease states have been attributed to either a specific or nonspecific deficit in IL-1 production or response (14). Watson, et al. (17) showed that adherent cells from PBML of six untreated BT/TT and two untreated BB patients produced IL-1 in quantities within the normal range when stimulated with LPS or phorbol myristate acetate (PMA). However, adherent cells from 5 of 13 BL/LL patients (39%) did not produce detectable IL-1 in response to LPS or PMA. It was also shown that the numbers of adherent cells recovered from the PBML of BL/LL patients were significantly greater than those from normal controls, while BB or TT patients showed no differences. Using PBML from four lepromatous leprosy patients, Harcgewoin, et al. (4) showed that IL-1 production was normal when stimulated with M. leprae and BCG. It was very difficult to evaluate and interpret their results when PBML was used in the study due to the differences in quantities and the adherent properties of monocytes from each individual.
We have carefully studied the production of IL-1 from monocytes of various types of leprosy patients and normal subjects. Using the same technique for isolation of monocytes from leprosy patients and normal subjects, we noted that the adhering properties of monocytes from various types of leprosy patients were quite different from normal subjects. There was a lower percentage of cells adhering to the plastic plates in BT, BB, BL and LL patients than in normal subjects; however, no differences were noted between TT patients and normal controls. From our data, one can see that if one studies IL-1 production from the monocytes of leprosy patients, using the surface adherence technique for isolation and separation of monocytes, different concentrations of monocytes will be obtained. Therefore, the appropriate techniques and corrections have to be used in order to get the same concentration of monocytes from different subjects. Using 2 x 105/ml of monocytes from various types of leprosy patients and normal controls for IL-1 production, lower levels of IL-1 were obtained in the LL, BL, BB and BT patients than in normal controls; no differences were observed between TT patients and normal controls.
IFN-γ is an important mediator of both antimicrobial activity and hydrogen peroxide secretion in mononuclear phagocytes (12). Defective IFN-γ production was found in 17 out of 18 BL/LL patients in response to M. leprae antigen or mitogen ConA, while BB patients and a few BT/TT patients displayed intermediate responses and most BT/ TT patients showed vigorous IFN-γ production in response to both antigen and mitogen (13). Another study showed that most of the BL/LL patients produced low levels of IFN-γ in response to antigens of both M. leprae and BCG. while producing high levels of IFN-γ in response to ConA. On the other hand, BT/TT patients produced high levels of IFN-γ in response to M. leprae, BCG, and ConA (8). Our study shows that there were no differences in IFN levels between leprosy patients and normal controls when PBML were stimulated with PHA or ConA. However, when PPD was used as the stimulating agent, significantly lower levels of IFN were observed in treated and untreated BL/LL and treated BT/TT patients as compared to normal controls; the number of untreated BT/TT cases was too low for statistical comparison.
The mechanisms behind the failure of leprosy patients' monocytes and lymphocytes to produce IL-1 and IL-2 and IFN in response to LPS and PPD, respectively, requires further investigation and evaluation.
Acknowledgment. This research was supported by a USAID/PSTC Program Grant no. 936-5542-G-00-5056-00.
REFERENCES
1. Billieu, A., van Damme, J., Opdenakker, G., Fibbe, W. E., Falkenburg, J. H. and Content, J. Interleukin 1 as a cytokine inducer. Immuno-biology 172(1986)323-335.
2. Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21 Suppl. 97(1968)77-89.
3. Haregewoin, A., Godal, T.. Mustafa, A. S., Be-lehu, A. and Yemaneberhan. T. T cell conditioned media reverse T cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
4. Haregewoin, A., Mustafa, A. S., Helle, I., Waters, M. F. R.. Leiker, D. L. and Godal, T. Reversal by interleukin-2 of the T cell unresponsiveness of lepromatous leprosy to Mycobacterium leprae. Immunol. Rev. 80(1984)77-86.
5. Horwitz, M. A.. Levis, W. R. and Cohn. Z. A. Defective production of monocyte activating lym-phokines in lepromatous leprosy. J. Exp. Med. 159(1984)666-678.
6. Jeevan. A. and Asherson, G. L. Recombinant interleukin-2 limits the replication of Mycobacterium lepraemurtum and Mycobacterium bovis BCG in mice. Infect. Immun. 56(1988)660-664.
7. Kaplan. G. and Cohn. Z. A. The immunobiology of leprosy. Int. Rev. Exp. Pathol. 28(1986)45-78.
8. Kaplan, G., Weinstein, D. E., Steinman, R. M., Levis, W. R., Elvers, U., Patarroyo, M. E. and Cohn, Z. A. An analysis of in vitro T cell responsiveness in lepromatous leprosy. J. Exp. Med. 162(1985)917-929.
9. Makonkawkeyoon. S. and Kasinrerk, W. In vitro suppression of interleukin 2 production by Mycobacterium leprae antigen. Clin, Exp. Immunol. 76(1989)398-403.
10. Malkovsky, M.. Sondel, P. M., Strober, W. and Dalgi.eish. A. G. The interleukins in acquired disease. Clin. Exp. Immunol. 74(1988)151-161.
11. MoHagheghpour, N.. Gelber, R. H., Larrick, J. W., Sasaki. D. T., Brennan, P. J. and Engle-man, E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J. Immunol. 135(1985)1443-1449.
12. Nathan, C. F.. Murray, H. W., Wiebe, M. E. and Rubin, B. Y. Identification of intcrferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158(1983)670-689.
13. Nogueira, N., Kaplan. G.. Levy, E., Sarno, E. N., Kushner, P., Granelli-Piperno, A., Vieira, L., Colomer Gould, V., Levis, W., Steinman, R., Yip, Y. K. and Cohn, Z. A. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J. Exp. Med. 158(1983)2165-2170.
14. Oppenheim. J. J.. Kovacs. E. J.. Matsushima, K. and Durum. S. K. There is more than one interleukin 1. Immunol. Today 7(1986)45-56.
15. Ottenhoff, T. H. M., Elferink, D. G. and deVries, R. R. P. Unresponsiveness to Mycobacterium leprae in lepromatous leprosy in vitro: reversible or not? Int. J. Lepr. 52(1984)419-422.
16. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
17. Watson. S., Bullock, W., Nelson, K., Schauf, V., Gelber, R. and Jacobson, R. Interleukin 1 production by peripheral blood mononuclear cells from leprosy patients. Infect. Immun. 45(1984)787-789.
1. Ph.D., Department of Clinical Immunology. Faculty of Associated Medical Sciences; Chiang Mai University, Chiang Mai 50002, Thailand.
2. M.S., Department of Clinical Immunology. Faculty of Associated Medical Sciences; Chiang Mai University, Chiang Mai 50002, Thailand.
3. M.S., Department of Microbiology, Faculty of Medicine; Chiang Mai University, Chiang Mai 50002, Thailand.
4. M.D., Ph.D., Department of Microbiology, Faculty of Medicine; Chiang Mai University, Chiang Mai 50002, Thailand.
5. U.S., Section of Microbiology and Immunology, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai 50002, Thailand.
Received for publication on 30 May 1989.
Accepted for publication in revised form on 8 November 1989.