- Volume 58 , Number 2
- Page: 319–27
Heterogeneity ot serological responses in paucibacillary leprosy - differential responses to protein and carbohydrate antigens and correlation with clinical parameters
ABSTRACT
We have examined the serological responses of 154 untreated paucibacillary (PB) leprosy patients to two carbohydrate and one protein antigens of Mycobacteriuni leprae. There was a heterogeneous response with 20% of PB patients having IgM anti-PGL-I antibodies and a similar proportion with IgG anti-LAM antibodies, while 33% had antibodies to the M. leprae-specific epitope on the 35-kDa protein. There was overlap in the responses such that 43% of the patients were seropositive in one of the two M. leprae-specific assays, while 49% were positive in any assay. There was a gradation in seropositivity with increasing extent of disease for each of the clinical parameters measured. Those with established disability at the time of presentation were more likely to be seropositive in each assay.RÉSUMÉ
Chez 154 malades atteints de lèpre paucibacillaire (PB), et non traités, on a étudié les réponses sérolo-giques à deux hydrates de carbone et à un antigène protéinique de Mycobacteriuni leprae. On a enregistré une réponse hétérogène; en effet, 20% des malades pau-cibacillaires avaient des anticorps IgM anti-PGL-1, une proportion semblable présentait des anticorps IgG anti-LAM, alors que 33% révélaient la présence d'anticorps à l'épitope spécifique pour M. leprae de la protéine 35-kDa. On a observé un chevauchement dans les réponses; 43% des malades étaient séropositifs pour l'un ou l'autre de ces deux titrages spécifiques pour M. leprae, alors que 49% étaient positifs pour les deux. On a enregistré de plus une augmentation graduelle de la séropositivité en relation avec l'étendue de la maladie, et ceci pour chacun des paramètres cliniques qui ont été mesurés. Les malades ayant des déformités établies au moment de l'étude avaient davantage de chances d'être séropositifs pour chacune des épreuves.RESUMEN
Se examino la respuesta serológica de 154 pacientes con lepra paucibacilar (PB) a dos carbohidratos y un antígeno protéico del Mycobacterium leprae. Se encontró una respuesta heterogénea donde 20% de los pacientes PB tuvieron anticuerpos IgM anti-GLF-I y una proporción similar tuvo anticuerpos IgG anti-LAM; 33% de los pacientes tuvieron anticuerpos contra el epitope de 35 kDa específico del M. leprae. Hubo tal sobreposición en las respuestas que el 43% de los pacientes fueron seropositivos en uno de los dos ensayos específicos para M. leprae, mientras que el 49% de los mismos fueron positivos en cualquiera. Hubo un incremento en la seropositividad al aumentar la extensión de la enfermedad. Aquellos pacientes con más alteración orgánica al inicio del estudio fueron más propensos a resultar seropositivos en cualquiera de los ensayos.There is a wide range in the serological responses of leprosy patients to Mycobacterium leprae, and this in part mirrors the clinical spectrum of the disease (8,14). Lep-romatous patients have a strong humoral response to many mycobacterial antigens, while fewer patients at the tuberculoid pole of the disease have detectable antibodies. Recent serological studies have focused on the definition of species-specific carbohydrate and protein antigenic determinants of M. leprae and the strong responses in lep-romatous patients (2-5,7,17,25). A significant group of tuberculoid patients do, however, have antibody responses to crude and specific antigen preparations (18,19,24), although the relative importance of carbohydrate and protein antigens in individual patients is unclear (10).
For the implementation of multidrug therapy (MDT) the World Health Organization Expert Committee has recommended that patients with polar tuberculoid (TT) and borderline tuberculoid (BT) leprosy be assigned to the paucibacillary (PB) treatment category and so receive 6 months' therapy with two drugs (21). Recently, this was revised so that BT patients with a positive skin smear are now assigned to the multibacillary (MB) treatment category and receive treatment for at least 2 years with three drugs (22). There is still a wide spectrum of disease in the PB category and because of a variable response to WHO-MDT in some BT patients (11), attempts have been made to divide BT patients on clinical grounds into those with more extensive disease requiring MB-MDT and those with limited disease for whom PB-MDT is sufficient (20). The validity of this division is uncertain, and there have been few comparisons of the clinical and immunological parameters in PB patients diagnosed under field conditions. We therefore decided to examine the serological responses ofa group of untreated PB patients to three defined antigens. This was to determine if the response to carbohydrate and protein antigens is similar in the same PB patients, whether the antibody response parallels clinical measures of the duration and the extent of the disease at the time of diagnosis, and whether the serological profile can assist in the assignment of BT leprosy patients to PB or MB categories.
MATERIALS AND METHODS
Patients
The patient group consisted of 154 self-referred, previously untreated patients with TT or BT leprosy diagnosed on clinical and bacteriological grounds according to the Ridley-Jopling classification (16) in three leprosy clinics in Nepal. From data collected prospectively, the duration of the disease was estimated by the length of history before presentation and the cumulative disability index (DI) on a scale of 0 to 18 which was the sum of changes in the four limbs and both eyes, each ranked on a scale of 0 to 3 (21). The clinical extent of disease was assessed by the number of skin patches, the number of enlarged nerves, and the number of body areas involved with skin or neural disease (with a maximum of 9, consisting of 4 for the trunk divided sagittally anterior and posterior, 4 for the limbs, and 1 for the head). The mean bacterial index (BI) was calculated from slit-skin smears at four sites, including one clinical lesion. Serum samples were collected and stored in 0.001% sodium azide at - 20°C until testing. Control sera from healthy Nepali medical and nursing students were kindly provided by Mr. B. Pokherel, Institute of Medicine, Kathmandu, Nepal.
Serological assays
IgM anti-PGL-I antibodies. IgM anti-phenolic glycolipid (PGL-I) antibodies (Ab) were measured by an enzyme-linked immunosorbent assay (ELISA) using the gly-coconjugate disaccharide-bovine serum albumin (dBSA; provided by IMMLEP, World Health Organization) (3). Briefly, wells of Cooke microtiter trays (Dynatech, Alexandria, Virginia, U.S.A.) were coated with 100 μl dBSA. 250 ng/ml, in 0.05 M sodium carbonate buffer, pH 9.6, blocked with 200 μl 1.0% w/v BSA and incubated with 100 μl patient sera diluted 1:300 in 10% normal goat serum (NGS) in phosphate buffered saline (PBS). After washing with PBS 0.05% Tween 20 (PBS/T), 100 μl goat anti-human IgM-peroxidase conjugate (Cappel Laboratories, West Chester, Pennsylvania, U.S.A.) diluted 1:4000 in 10% NGS/PBS was added before washing and incubation with 100 μl 0.4 g/l O-phenylene-diamine (Sigma Chemical Company, St. Louis. Missouri, U.S.A.) in 0.05 M citrate phosphate buffer, pH 5.0, containing 0.006% hydrogen peroxide. The reaction was stopped at 10 min by the addition of 100 μl of 2.5 M sulfuric acid, and the trays were read with a Multiskan Reader (Flow Laboratories, Irvine, Scotland). Samples with an absorbance at 492 nm greater than 0.199 (the mean absorbance plus three standard deviations of 91 healthy Nepali control subjects) were considered positive. Known positive and negative controls were included on each tray. The variation between assays was less than 10%.
IgG anti-LAM. IgG anti-lipoarabino-mannan (LAM) antibodies were measured by an ELISA using M. tuberculosis LAM as antigen (kindly provided by Dr. P. J. Bren-nan, Colorado State University, Fort Collins, Colorado, U.S.A.) at a concentration of 1 μg/ml and patient sera at a 1:1000 dilution (9), Samples with an absorbance at 492 nm greater than 0.419 (mean plus three standard deviations of 100 controls) were considered positive.
Antibodies to M. leprae-specific 35-kDa protein. Antibodies to the M. leprae-specific 35-kDa protein were detected by a monoclonal antibody (ML-04) inhibition ELISA (17). Round-bottom microtiter trays (Nunc, Kamstrup, Denmark) were coated with 50 μl M- leprae sonicate (10 μg/ml, provided by IMMLEP), blocked with 3.0% w/v BSA/PBS, and incubated with 25 μl of the sera diluted 1:10, 1:100, 1:1000, 1: 10,000 in 1.0% BSA/PBST for 1 hr. The 25 μl ML-04-peroxidase conjugate was added for 2 hr. The trays were then washed, and O-phenylenediamine substrate was added for 30 min. Positive control wells were incubated with conjugate without inhibiting scrum. The serum titer causing 50% inhibition of ML-04 binding to M. leprae sonicate (ID 50) was calculated, and titers greater than 10 were considered positive. All of the 56 control sera tested were negative.
Statistical analysis
The significance of differences in sero-positivity between patient groups was tested with the chi-squared statistic using Yates' correction. The coefficients of correlations between the three assays in the same subjects were determined by Pearson's correlation coefficient.
RESULTS
Serological responses
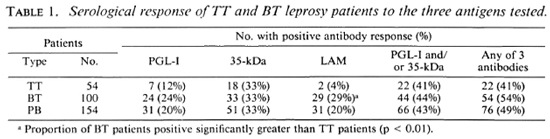
There were 106 male and 48 female patients with an age distribution of 7 to 68 years. One hundred of the patients were classified as having BT leprosy and 54 had TT leprosy. For the combined group of PB patients, 20% had IgM anti-PGL-I antibodies. 33% had anti-35-kDa antibodies, and 20% had IgG anti-LAM antibodies. There were no differences in seropositivity for the three antigens between males and females. A higher proportion of BT patients had anti-PGL-I and anti-LAM antibodies than did TT patients (Table 1). Forty-nine percent of the PB sera were positive for one of the three antibodies tested and 43% were positive for either or both of the M. leprae-specific antibodies (IgM anti-PGL-I and anti-35-kDa antibodies).
Comparison with clinical parameters
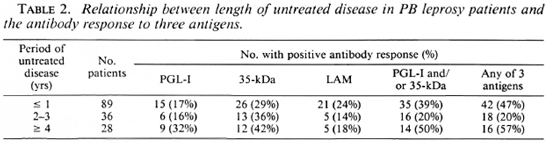
Duration of disease before presentation. The majority of patients had active disease for less than 1 year before presentation. There was a trend for the proportion of IgM anti-PGL-I-antibody-positive patients to increase with more long-standing disease, but this was not significant (Table 2).
Paucibacillary patients with established disability at the time of diagnosis were more likely to be IgM anti-PGL-I-antibody positive (33% vs 10%, p < 0.01); anti-35-kDa-antibody positive (49% vs 19%, p < 0.01); IgG anti-LAM-antibody positive (28% vs 13%, p < 0.05); or positive to any antigen (66% vs 34%, p < 0.01) than those without disability. With increasing disability there was a progressive increase in seropositivity for each assay (Fig. 1).
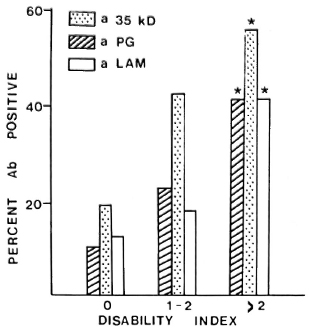
Fig. 1. Relationship between antibody positivity for the three antigens and cumulative disability index; * = significant increase in proportion seropositive, p < 0.05.
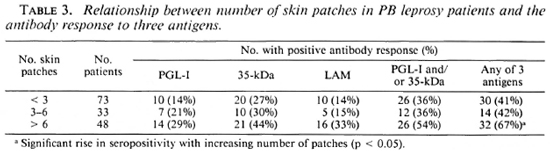
Clinical extent of disease. There was a trend for a higher proportion of patients with more than six patches to be seropositive for each assay, but these changes were not significant except where the three serological results were combined (Table 3).
A similar pattern was seen in those with nerve involvement. As more nerves were affected the antibody-positivity rate increased (Table 4), and this increase was significant for IgG anti-LAM antibodies (p < 0.01) and if any antibody assay was positive (p < 0.01).
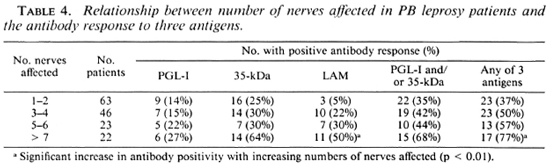
When the clinical extent was estimated by the number of body areas with skin patches and/or enlarged nerves, there was also an increase in antibody positivity as more areas were involved (Table 5), and this change was most marked for anti-LAM antibodies (p < 0.05) and for a combination of the assays (p < 0.01). When those with one or two areas affected or more than two areas were compared, the increase in scropositivity was also significant for IgG anti-LAM (p < 0.01) and a combination of the three assays (p < 0.05).
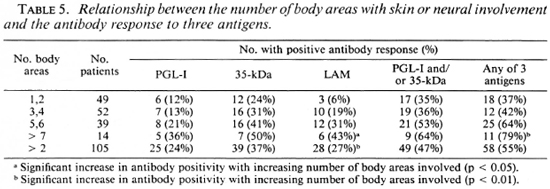
The majority of patients (146) were skin-smear negative. The other patients had BT leprosy with a smear-positive skin lesion (mean BI 0.25+ to 2 +), and a minority of these were seropositive (38% anti-PGL-I; 50% anti-35-kDa Ab and 13% anti-LAM Ab positive). These were not significantly different than the seropositivity rates in smear-negative PB patients.
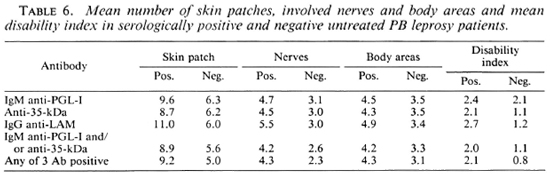
The extent of apparent disease in seropositive and seronegative PB patients is shown in Table 6. Although the differences were not statistically significant, the mean numbers of skin patches, involved nerves, involved body areas and the mean maximum disability index are greater in seropositive patients. The difference was not greater when a combination of assays was used to define seropositivity.
Correlations between antibody assays
PB patients who had anti-35-kDa antibodies were likely to have IgM anti-PGL-antibodies (p < 0.05) and IgG anti-LAM antibodies (p < 0.01). However, the concordance was not complete. A large group of PB patients with anti-peptide antibodies had no detectable responses to PGL-I (Fig. 2) or LAM (Fig. 3). There was a similar relationship between patients seropositive for anti-PGL-I and anti-LAM antibodies (p < 0.01), but 16 of the 29 anti-LAM-positive patients were anti-PGL-I negative (Fig. 4).
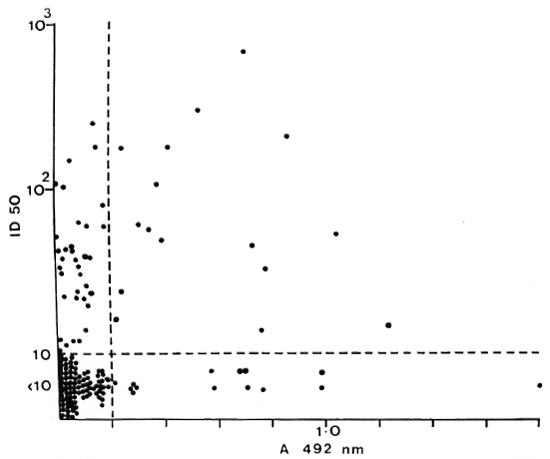
Fig. 2. Correlation between anti-35-kDa antibodies and IgM anti-PGL-I antibodies in individual untreated PB patients; r = 0.237, p < 0.01.
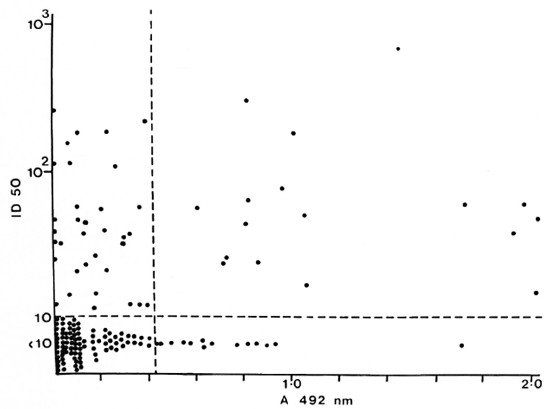
Fig. 3. Correlation between anti-35-kDa antibodies and IgG anti-LAM antibodies in individual untreated PB patients; r = 0.264, p < 0.01.
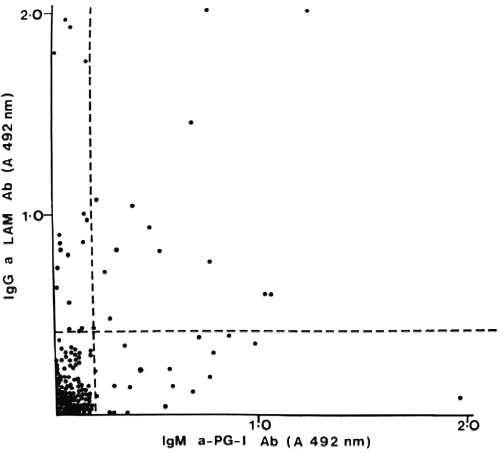
Fig. 4. Correlation between IgM anti-PGL-I and IgG anti-LAM antibodies in individual untreated PB patients; r = 0.300, p < 0.001.
In individual patients there was a weak correlation between the antibody levels to the individual antigen: PGL-I and LAM antibodies, p < 0.001; PGL-1 and 35-kDa antibodies, p < 0.05; 35-kDa and LAM antibodies, p < 0.01. This was true for both the TT and BT patient groups.
DISCUSSION
This group of PB leprosy patients were heterogeneous in their serological responses to the three antigens tested. A higher proportion responded to the M. leprae-specific epitope on the 35-kDa protein than to either of the carbohydrate determinants: the dominant terminal disaccharide of the PGL-I molecule presented by the neoconjugate (3,7) or the alpha-1-5-linked arabino-furanosyl epitope on the native LAM molecule (8,9). Further, a similar proportion of BT and TT patients responded to the 35-kDa protein; whereas there was a gradation of responses between the BT and TT patients for both of the carbohydrate determinants (Table 1). Earlier studies had suggested that cell-wall carbohydrates were the dominant B-cell antigens in BT patients (10); however, it is clear that different PB patients respond to protein or carbohydrate determinants. Therefore, a combination of assays defined a larger group of seropositive patients than any single assay.
The seropositivity rates for both anti-PGL-I and anti-LAM antibodies in this cohort was lower than that previously reported in small groups of BT and TT patients (1-3,5,7,13,13). This may be in part due to our use of endemic control sera from subjects exposed to pathogenic and environmental mycobacteria to define the positive cut-off value as the mean control value plus three standard deviations. This increased the specificity of the assays, but with a possible reduction in sensitivity. An additional factor may be the variability of PB patients in individual studies. For instance, patients with a longer history of untreated disease are more likely to be seropositive (Table 2, Fig. 1), and the inclusion of more such patients in a group will give a higher seropositivity rate. Similarly, if patients with more extensive BT leprosy, especially those with symptoms of neural disease (Table 4), are included, the seropositivity rates will be higher. This may occur if the patients sampled are at a referral institution rather than in a field setting.
The seropositive and seronegative patients did not clearly segregate into those with more and less extensive disease. Rather, there was a gradation in seropositivity from those with limited disease to those with more widespread disease, whether defined by the number of patches, enlarged nerves, or body areas involved (Tables 3, 4, 5). This rise in seropositivity occurred for each antigen but was most apparent for the IgG anti-LAM antibodies. However, some tuberculoid patients with disease limited to a single nerve or patch were still seropositive and, conversely, others with more extensive disease were seronegative. Therefore, although the proportion of seropositive parallels the clinical extent of apparently tuberculoid disease in this group of patients, there are other systemic immunoregulatory factors controlling the serological response in individual patients.
Some treatment programs have advocated the use of broad clinical parameters to define which patients should receive PB- or MB-MDT rather than use the Ridley-Jop-ling scale as proposed by the WHO Expert Committee on Leprosy (22,23). For instance, van Brakel and co-workers (20) recommended that PB patients with more than two body areas involved should receive MB-MDT. In this study, the majority of PB patients defined by these criteria were still seronegative and lacked the serological responses typical of MB leprosy (3,7). Therefore, the antibody response alone does not appear to assist in defining a subgroup of PB patients who may require MB therapy. Although these recommendations are easily applied in field situations where clinical classification and skin smears may not be reliable, the criteria may not reflect true differences in bacillary load or clinical outcome.
Disability caused by motor and sensory nerve damage is the most important factor for the individual leprosy patient. Patients with more long-standing disease and delayed presentation have more established nerve damage and thus higher disability indices on diagnosis. Interestingly, such patients with established disability were more likely to respond to each of the antigens tested than patients without disability (Fig. 1). This was true for both TT and BT patients, suggesting that the longer a PB patient has established disease prior to treatment, the more likely the patient is to become antibody positive. The same trend was observed for M. leprae-specific antibodies using the self-reported length of history (Table 2). It is unknown if antibody-positive PB patients without established disability at the time of diagnosis are more likely to develop disability during MDT, but this group of patients will be followed during MDT to determine this.
Monitoring the effects of chemotherapy in PB leprosy can be difficult. Skin lesions can persist after effective antibacillary therapy (11) as will neurological damage and disability, and the vast majority are skin-smear negative. Therefore, in the PB patients who are seropositive to one of the three antigens tested, changes in the antibody levels may be a useful tool for assessing response. Anti-PGL-I antibody levels have been shown to fall in highly positive MB patients during drug treatment (6), while in cross-sectional studies, antibody levels are lower in treated than untreated PB patients (1-4). Therefore, in individual seropositive PB patients, changes in their modest antibody levels may be a useful adjunct to clinical assessment in monitoring chemotherapy.
Acknowledgment. The Mycobacterial Research Laboratory is supported financially by The Leprosy Mission (International). We thank Dr. R. J. W. Rees for the provision of M. leprae antigens through the IMMLEP component of the WHO/UNDP/World Bank Tropical Diseases Research Programme. We also thank Dr. J. Ivanyi for providing ML-04 conjugate and Dr. P. J. Brennan for providing LAM (prepared under NIH contract A1-52582). This study was dependent on the willing cooperation of the doctors and nursing staff at Anandaban Leprosy Hospital, Tansen Hospital and Green Pastures Leprosy Hospital. We are grateful to Mrs. Beulah Jayakumar for her secretarial assistance.
REFERENCES
1. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot. F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. BRETT, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipoid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
3. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
4. Britton, W. J., Garsia. R. J. and Basten. A. Serological response to phenolic glycolipid of Mycobacterium leprae in Australian and Nepali leprosy patients. Aust. N.Z. J. Med. 17(1987)568-573.
5. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in the serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
6. Douglas. J. T., Hirsch, D. S., Fajardo, T. T., Cellona. R. V., Abalos, R. M., dela Cruz, E. C, Madarang, M. G., de Wit, M. Y. L. and Klatser, P. R. Evaluation of Mycobacterium leprae antigens in the serological monitoring of dap-sone resistant lepromatous leprosy patients in Cebu, Philippines. Lepr. Rev. 60(1989)8-19.
7. Fujiwara. T., Hunter, S. W., Cho, S.-N., Aspi-nall, G. O. and Brennan, P. J. Chemical synthesis and serology of disaccharides and trisac-charides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
8. Gaylord, H. and Brennan, P. J. Leprosy and the leprosy bacillus; recent developments in characterization of antigens and immunology of the disease. Annu. Rev. Microbiol. 41(1987)645-675 (139 refs).
9. Gaylord, H.. Brennan, P. J., Young, D. B. and Buchanan, T. M. Most Mycobacterium leprae carbohydrate reactive monoclonal antibodies are directed to lipoarabi nomannan. Infect. Immun. 55(1987)2860-2863.
10. Harboe, M., Closs, O., Reitan, L. J. and Draper, P. Demonstration of antibodies reacting with different determinants on Mycobacterium leprae antigen 7. Int. J. Lepr. 49(1981)147-158.
11. Katoch, K., Ramu. G., Ramanathan, U. and Desikan, K. V. Comparison of three regimens containing rifampin for treatment of paucibacil-lary leprosy patients. Int. J. Lepr. 55(1987)1-8.
12. Levis, W. R., Meeker. H. C, Schuller-Levis, G. B., Gillis. T. P., Marino, L. J. and Zabriskie, J. Scrodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J. Clin. Microbiol. 24(1986)917-921.
13. Levis, W. R., Meeker, H. C, Schuller-Levis, G. B., Sersen, E.. Brennan, P. J. and Fried, P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J. Infect. Dis. 56(1987)763-769.
14. Melsom, R. Scrodiagnosis of leprosy; the past, the present and some prospects for the future. Int. J. Lepr. 51(1983)235-252.
15. Mwatha, J., Moreno, C, Sengupta, U., Sinha, S. and Ivanyi. J. A comparative evaluation of serological assays for leproniatous leprosy. Lepr. Rev. 59(1988)195-199.
16. Ridley, D. S. and Jopling. W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
17. Sinha, S., Sengupta, U., Ramu, G. and Ivanyi, J. Serological survey of leprosy and control subjects by a monoclonal antibody based immunoassay. Int. J. Lepr. 53(1985)33-38.
18. Touw, J., Langendijk, E. M., Stoner, G. L. and Belehu, A. Humoral immunity in leprosy: immunoglobulin G and M antibody responses to Mycobacterium leprae in relation to various disease patterns. Infect. Immun. 36(1982)885-892.
19. Touw, J., Langendijk, E. J. M., van Diepen, T. W., Harboe, M. and Belehu, A. Relation between anti-Mycobacterium leprae antibody activities and clinical features in borderline tuberculoid (BT) leprosy. Int. J. Lepr. 51(1983)305-31 1.
20. van Brakel, W., Kist, P., Nobel, S. and O'Toole, L. Relapse after MDT. Lepr. Rev. 60(1989)81-89.
21. WHO Expert Committee on Leprosy. Fourth Report. Geneva: World Health Organization, 1970. Tech. Rep. Ser. 459.
22. WHO Expert Committee on Leprosy. Sixth Report. Geneva: World Health Organization. 1988. Tech. Rep. Ser. 768.
23. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization. 1982. Tech. Rep. Ser. 675.
24. Yoder, L., Naafs. B., Harboe, M. and Bjune, G. Antibody activity against Mycobacterium leprae antigen 7 in leprosy: studies on variation in antibody content throughout the spectrum and on the effect of DDS treatment and relapse in BT leprosy. Lepr. Rev. 50(1979)113-121.
25. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. B.App.Sc; Mycobacterial Research Laboratory. Anandaban Leprosy Hospital. P.O. Box 151, Kathmandu, Nepal.
2. Ph.D.. F.R.A.C.P., F.R.C.P., Mycobacterial Research Laboratory. Anandaban Leprosy Hospital. P.O. Box 151, Kathmandu, Nepal.
3. M.Sc; Mycobacterial Research Laboratory. Anandaban Leprosy Hospital. P.O. Box 151, Kathmandu, Nepal.
4. M.D., M.P.R.Ch., Mycobacterial Research Laboratory. Anandaban Leprosy Hospital. P.O. Box 151, Kathmandu, Nepal.
5. R.N., Tansen Hospital, Palpa, Nepal.
6. M.B., M.P.H., Public Health Division, Ministry of Health. Teku, Kathmandu. Nepal.
Reprint requests to Paul W. Roche.
Received for publication on 21 July 1989.
Accepted for publication in revised form on 16 January 1990.