- Volume 58 , Number 2
- Page: 334–41
Natural killer-cell-mediated and antibody-dependent cellular cytotoxicity in leprosy
ABSTRACT
We have assessed the natural killer (NK) cell-mediated cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) in the peripheral blood lymphocytes (PBL) f rom untreated lepromatous leprosy (LL) patients, LL patients on multidrug therapy (MDT) with favorable responses (MDT-R), LL patients clinically classified as nonresponders to MDT (MDT-NR), treated tuberculoid leprosy (TT) patients, and healthy donors. NK cytotoxicity was modulated by treating the PBL with recombinant intcr-feron-alpha (IFN-α) and recombinant in-terleukin-2 (IL-2).The mean percent NK cytotoxicity of untreated LL patients (15 ± 3), treated MDT-R patients (20 ± 4), and treated MDT-NR patients (12 ± 4) was significantly lower than that of TT patients (39 ± 6) and healthy donors (37 ± 5). Treatment of effectors with IL-2 or IFN-α enhanced NK cytotoxicity in 5 of 6 untreated LL patients, 6 of 6 treated MDT-R LL patients, 4 of 5 and 3 of 5 treated MDT-NR LL patients, respectively, and 5 of 8 and 3 of 8 treated TT patients, respectively.
Although PBL f rom TT patients showed initial NK activity comparable to that of healthy donors, fewer TT patients showed modulation of NK activity by IL-2, and IFN-α to a lesser extent. The ADCC activity was lower in untreated LL patients compared to treated patients, while TT patients had normal ADCC activity. The results indicate that although LL patients show lowered spontaneous cytotoxicity, it can be modulated favorably by lymphokines.
RÉSUMÉ
On a evalué la cytotoxicité naturelle à médiation cellulaire (NK), ainsi que la cytotoxicité cellulaire sous la dépendance des anticorps (ADCC) dans les lymphocytes du sang périphérique (PBL), chez des malades atteints de lèpre lépromateuse non traitée (LL), des sujets LL soumis à la polychimiothérapie (PCT) et répondant favorablement à celle-ci (MDT-R), des patients LL classifiés cliniquement comme non répondants à la polychimiothérapie (MDT-NR), des individus atteints de lèpre tuberculoïde et traités (TT), et des donneurs sains. La cytotoxicité NK était modulée lorsqu'on traitait les lymphocytes par de l'interferon-alpha obtenu par recombinaison génétique (IFN-α) et par de l'interleukine-2 (IL-2) également obtenue par recombinaison.La cytotoxicité moyenne NK exprimée en pourcentage était significativement plus basse chez les malades LL non traités (15 ± 3), chez les sujets MDT-R traités (20 ± 4), et chez les patients traités M DT-NR (12 ± 4), par rapport aux valeurs observées chez les sujets TT (39 ± 6) et chez les donneurs sains (37 ± 5). Le traitement des lymphocytes par IL-2 ou par IFN-α a entraîné une augmentation de la cytotoxicité NK chez 5 des 6 patients LL non traités, chez tous les six malades lépromateux MDT-R traités, chez 3 des 5 sujets LL traités MDT-NR. et respectivement 5 des 8 et 3 des 8 malades TT traités.
Encore que les lymphocytes du sang périphérique, recueillis chez les malades TT, aient démontré une activité NK initiale comparable à celle des donneurs sains, un nombre inférieur de ces sujets TT a témoigné d'une modulation de l'activité NK par IL-2, et dans une moindre mesure par IFN-α.
L'activité ADCC était plus faible chez les malades LLnon traités, par comparaison avec les malades traités, alors que les sujets TT présentaient une activité ADCC normale.
Ces résultats démontrent que malgré qu'elle soit abaissée chez les malades LL, la cytotoxicité spontanée peut être modulée dans un sens favorable par les lymphokines.
RESUMEN
Se analizó la citotoxicidad celular mediada por células asesinas naturales (NK) y la citotoxicidad celular dependiente de anticuerpo (CCDA) en los linfocitos desangre periférica (LSP): de pacientes lepromatosos (LL) sin tratamiento, de pacientes LL en tratamiento con múltiples drogas (TMD) y respuestas favorables (TMD-R), de pacientes LL no respondedores al tratamiento (TMD-NR), de pacientes tuberculides tratados (TT), y de donadores sanos. La citotoxicidad NK fue modulada por tratamiento de los LSP con interferon alfa (IFN-α) e interleucina-2 (IL-2) recombinantes.Los porcentajes promedio de citotoxicidad por NK en los pacientes LL no tratados (15 ± 3), en los pacientes TMD-R (20 ± 4), y en los pacientes TMD-NR (12 ± 4) fueron significativamente más bajos que aquellos en los pacientes TT (39 ± 6) y en los donadores normales (37 ± 5). El tratamiento de los efectores con IL-2 o con IFN-α incrementó la citotoxicidad NK en 5 de 6 pacientes LL no tratados, en 6 de 6 pacientes TMD-R, en 4 de 5 y en 3 de 5 pacientes LL TMD-NR, y en 5 de 8 y en 3 de 8 pacientes TT.
Aunque los LSP de los pacientes TT mostraron una actividad NK inicial comparable a la de los controles sanos, menos pacientes TT mostraron menor grado de modulación de la actividad NK por IL-2 e IFN-α. La actividad CCDA fue más baja en los pacientes LL no tratados que en los pacientes tratados; los pacientes TT tuvieron una CCDA normal. Los resultados indican que aunque los pacientes LL muestran una cilotoxi-cidad espontánea disminuida, ésta puede ser favorablemente modulada por linfocinas.
Natural killer (NK)-cell-mediated cytotoxicity and antibody-dependent cellular cytotoxicity (ADCC) are important host defense mechanisms against cancer (9,10). NK cells may also serve as potential effectors in microbial defense, particularly in viral diseases (20). It is suggested that NK-cell cytotoxicity is the first line of defense in viral infections, before the cytotoxic T lymphocytes are activated and clonally expanded (16). Recently, NK cells are shown to possess potent antibacterial activity against gram-negative and gram-positive bacteria(8), and against cells harboring intracellular bacteria (1).
Mycobacteria are obligate intracellular pathogens, residing in macrophages and Schwann cells. Therefore, cellular cytotoxic mechanisms may have an important role in limiting infection in mycobacterial diseases. Currently, much interest has been generated in studying the role of NK cells in mycobacterial diseases, especially leprosy (4-6,11) triggered by the observations on increased NK activity in reversal reactions (6) and decreased NK-cell-mediated killing in erythema nodosum leprosum (ENL) reactions (11). The histological evidence put forth by Kaplan, et al. (12) of the association of fragmented Mycobacterium leprae with NK/ LAK (lymphokine activated killer)-like cells at the delayed-type hypersensitivity (DTH) site induced by PPD in lepromatous leprosy patients, directly supports the role of NK cells in mycobacterial infections. The present investigations were aimed at studying the NK and ADCC activities of peripheral blood lymphocytes (PBL) from leprosy patients and the modulation of NK cytotoxicity by recombinant interleukin-2 (rIL-2) and recombinant intcrferon-alpha (rIFN-α).
MATERIALS AND METHODS
Patients and healthy donors. Leprosy patients included in our studies were from the outpatient clinics at Richardson Leprosy Hospital, Miraj, India (about 400 km from Bombay in western Maharashtra state) and Acworth Leprosy Hospital, Bombay, India. They were grouped as follows: Group A = clinically and histologically confirmed untreated, newly diagnosed lepromatous leprosy (LL) patients. Group B = LL patients undergoing multidrug therapy (MDT) for up to 2 years, showing clinical improvement (MDT-R). Group C = LL patients being treated for up to 4 years with MDT, not responding to the therapy (MDT-NR). These patients did not show any improvement in their bacterial index (BI) over the period of treatment. They had an average BI of 2.7 on the Ridley scale at the time of the experiments. Group D = tuberculoid leprosy (TT) patients undergoing treatment. Group E = laboratory personnel with no immediate past history of infection served as controls.
Separation of effector cells. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood on a Ficoll-Hypaque (FH; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) gradient. For removal of adherent cells, the PBMC were suspended in complete medium (CM) consisting of RPMI 1640 (GIBCO, Grand Island. New York, U.S.A.) supplemented with 10% human blood group AB serum, 4 mM glutamine, penicillin (100 U/ml), 2 mer-captoethanol (5 x 10-5 M), streptomycin (100 μg/ml), gentamycin (20 μg/ml),and plated at a density of 20 x 106 cells in 4 ml of CM for 2 hr at 37ºC in a humidified 5% CO2 atmosphere on glass petri dishes pre-coated with fetal calf serum (FCS; GIBCO). Nonadherent PBL were used as effectors in a 4-hr 51chromium release assay after overnight incubation in CM. Whenever the cell numbers were adequate, modulation experiments were performed. For this purpose, PBL were incubated overnight in four lots of medium alone, with IL-2, with IFN-α, and with the combination of IL-2 and IFN-α.
Treatment of NK effectors with modulating agents. The rIFN-α and the rIL-2, used as modulating agents, were kindly supplied as a gift by the Ernst Boehringer Institute of West Germany and by Hoffman-La Roche of Switzerland, respectively. The optimum doses for IL-2 and IFN-α capable of augmenting the NK cytotoxicity of lymphocytes from healthy donors were 50 U/l x 106 cells and 100 U/l x 106 cells, respectively, as assessed by conducting the dose-response studies. A mixture of 50 U IL-2 and 100 U IFN-α were also used to assess the combined effect of the agents to modulate the NK cytotoxicity.
Labeling of targets for NK and ADCC assays. NK targets (erythroleukemic cell line K562, 1 x 106 cells in 100 μl FCS) and ADCC targets [chicken red blood cells (CRBC) 20 x 106 cells in 100 μlFCS] were labeled with 100 μCiof Na2 51Cr04 (Bhabha Atomic Research Centre, Bombay, India, specific activity 15-20 mCi mg-1). After incubation at 37ºC in a water bath for 1 hr with intermittent shaking, the cells were washed three times with RPMI 1640 supplemented with 10% FCS, and resuspended so as to obtain 1 x 104 K562 cells or 1 x 105 CRBC/100 μ1 of the medium.
NK assay. The NK assay was performed as described earlier (7). One hundred μl of 51Cr-labeled targets (1 x 104 cells) and 100 μ1 of effector cells at different concentrations leading to effector-to-target (E:T) ratios of 50:1, 25:1, 12.5:1 were added in triplicate to wells of round-bottom microtiter plates (Nunclon; Nunc, Denmark). The plates were spun at 500 rpm for 5 min and incubated for 4 hr at 37ºC in a 5% humidified CO2 atmosphere. The assay was terminated by pelleting cells at 1000 rpm for 10 min. Cytotoxicity was assessed by counting the radioactivity in 100 μ1 of the supernatant from each well on a gamma counter. Spontaneous 51Cr release from targets was determined from wells in which 100 μl of labeled targets were incubated with 100 μl of medium alone. Spontaneous release ranged from 7%- 10% in these tests. The maximum release was assessed from supernatants of wells containing 1 x 104 target cells suspended in 100 μlof medium, lysed by the addition of 100 μl of 3% v/v Triton X-100. The percent cytotoxicity was calculated by the following formula:

ADCC assay. For ADCC, 50 μlof rabbit anti-CRBC serum at a dilution of 1:500 (predetermined subagglutinating dilution) was added to 50 μl of labeled CRBC (5 x 104) dispensed in round-bottom microliter plates. Fifty μl of effector cells were further added in triplicate to give the E:T ratio of 20:1. Spontaneous release in the presence of effectors alone, antiserum alone and medium alone were also determined. Spontaneous release did not exceed 2%-5% in these tests. Maximum release was attained by the addition of 50 μl 3% v/v Triton X-100 to 50 μl of CRBC targets in triplicate. The plates were incubated for 4 hr, and cytotoxicity was measured as described for the NK assays.
Statistical analysis. The results were evaluated for statistical significance by Student's t test.
RESULTS
NK and ADCC activities. As indicated earlier, leprosy patients were grouped as: A = untreated LL patients; B = treated MDT-R LL patients; C = treated MDT-NR LL patients; D = TT patients undergoing treatment. Group E represented healthy controls. The mean percent NK cytotoxicity was significantly reduced in all three groups of LL patients compared to TT patients and healthy controls when tested at all three E:T ratios (The Table). The lower range of NK cytotoxicity of all three LL groups was very low: 7/10 untreated LL patients, 5/11 MDT-R LL patients and 11/12 MDT-NR LL patients showed NK cytotoxicity lower than 19%, which was the lowest NK activity shown by healthy donors (Figs. 1-3). Although MDT-NR LL patients showed a mean percent NK activity lower than untreated and MDT-R LL patients, these differences were not statistically significant.
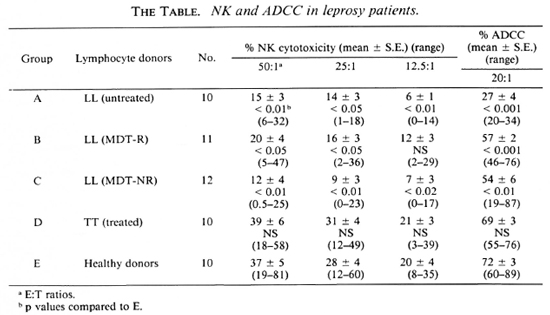
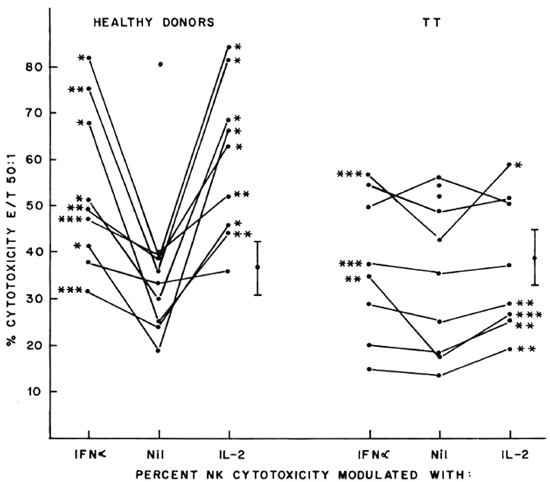
Fig. 1. Percent NK cytotoxicity of PBL from healthy donors (10) and TT patients (10) at E:T = 50:1 and modulation of NK cytotoxicity with IFN-α and IL-2. Effectors from 9 healthy donors and 8 TT patients were treated with the cytokines for modulation of NK cytotoxicity. Vertical bar represents mean ± S.E. of baseline NK cytotoxicity; * = p < 0.001; ** = p < 0.01; *** = p < 0.05.
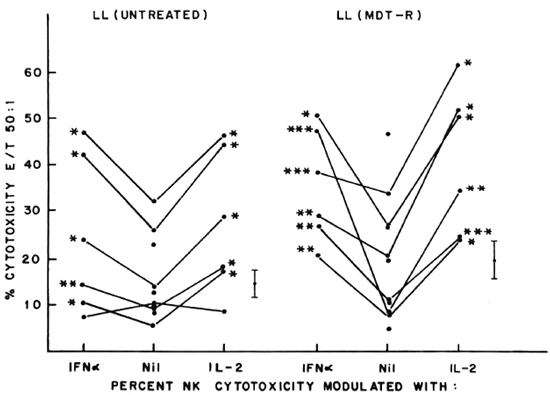
Fig. 2. Percent NK cytotoxicity of PBL from untreated LL patients (10) and treated MDT-R LL patients (1 1) at E:T = 50:1 and modulation of NK cytotoxicity with IFN-α and IL-2. Effectors from 6 untreated LL patients and 6 treated MDT-R LL patients were treated with the cytokines for modulation of NK cytotoxicity. Vertical bar represents mean ± S.E. of baseline NK cytotoxicity; * = p < 0.001; ** = p < 0.01; *** = p < 0.05.
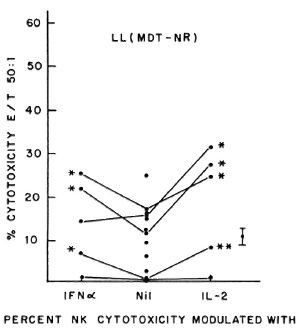
Fig. 3. Percent NK cytotoxicity of PBL from treated MDT-NR LL patients (12) at E:T = 50:1 and modulation of NK cytotoxicity of 5 treated MDT-NR LL patients with IFN-α and IL-2. Vertical bar represents mean ± S.E. of baseline NK activity; * = p < 0.001; ** = p < 0.01.
PBL from the three groups of LL patients (A, B and C) showed significantly low ADCC activity when compared with healthy donors and TT patients (The Table). ADCC of untreated LL patients was signficantly less than that of treated LL patients from both groups B and C. Again, the ADCC activity of lymphocytes obtained from TT patients and healthy donors was comparable.
Modulation of NK cytotoxicity. IL-2 (50 U/l x 106 lymphocytes), IFN-α (100 U/l x 106 lymphocytes), and a combination of both of these modulating agents at the same concentrations were used to treat the effectors overnight. The percent NK cytotoxicity of PBL from 8 out of 9 healthy donors could be significantly augmented with IL-2 and IFN-α (Fig. 1). When the range of percent increase in NK cytotoxicity was evaluated in each case, it varied from 9%-247% when modulated with IL-2 and 15%-172% when modulated with IFN-α; NK cytotoxicity of TT patients could be significantly modulated in 5 out of 8 cases with IL-2 (the range of percent increase being 18%-53%), and only in 3 out of 8 cases with IFN-α (range of percent increase 12%-97%, Fig. 1).
Five of the six untreated LL patients tested showed significant augmentation of NK cytotoxicity with IL-2 (range of percent increase 44%-203%) and IFN-α (range of percent increase 47%-77%). Similar significant modulation was oberved in all the 6 patients tested out of 11 MDT-R LL patients; the range of percent augmentation with IL-2 being 81%-301%, with IFN-α being 12%-451% (Fig. 2). From 12 MDT-NR LL patients, lymphocytes from 5 were tested for NK activity after treatment with lympho-kines. Four of these five showed significant augmentation of NK response with IL-2 treatment (percent increase 43%-152%) and 3 of the 5 with IFN-a treatment (percent increase 49%-110%, Fig. 3). The percent NK cytotoxicity achieved after augmentation with IL-2 or IFN-α of PBL from MDT-NR LL patients was below the baseline level of NK cytotoxicity of the healthy donors. The treatment of effectors with a combination of IL-2 and IFN-α showed augmentation of NK cytotoxicity comparable to that achieved by treatment with the single agent in all groups (data not shown).
DISCUSSION
In the lepromatous form of leprosy, patients exhibit a selective anergy to M. leprae antigens as assessed by in vivo and in vitro tests for cell-mediated immunity (2). However, the immune response to unrelated antigens appears to be intact in these patients (2). LL patients do not seem to be typically immunocompromised since they do not show increased incidence of cancer or opportunistic infections (11). Nevertheless, extensive multiplication of M. leprae is seen in the dermal macrophages of LL patients, and no known cellular effector mechanisms seem to be actively involved in destroying the cells harboring M. leprae. The role of NK cells in leprosy is uncertain. However, recent observations by Kaplan, et al. (12),reporting the inllux of non-T cytotoxic cells at the PPD-induced DTH site in LL patients, indicate the importance of the NK mechanism in the elimination of cells harboring M. leprae.
Very few studies have been reported on circulating NK cells in leprosy patients, their modulation with lymphokines, or on the ADCC effector mechanism. In our present studies, we have shown that LL patients, irrespective of their status of treatment and responses to treatment, show reduced NK and ADCC functions. These observations are in agreement with those reported by Converse and Bjune (6) and Chai and Lee (4). From our data, it is apparent that long-term MDT treatment, which reduced the bacillary load, did not alter the low NK status of untreated LL patients. Humphres, et al. (11) have also reported that dapsone/ prednisolone treatment did not influence the NK status of LL/ENL patients.
The lowered NK activity in those LL patients observed by us could not be attributed to monocyte-induced suppression since we have used a nonadherent cell population to study NK cytotoxicity. Large amounts of M. leprae-specific antibodies and immune complexes in the LL patients' circulation may have contributed to the impairment of NK activity, as suggested by Lee. et al. (15).
It is well established that NK activity can be modulated with lymphokincs (17). The results reported here show that it is possible to augment the compromised NK activity of LL patients with modulating agents such as IL-2 and IFN-α. The percent cytotoxicity achieved after augmentation was comparable, in most cases except the MDT-NR group, to the mean baseline NK cytotoxicity of healthy donors. These observations, together with those of Kaplan, et al. (12), indicate the clinical usefulness of NK cells.
It was interesting to note that the baseline NK and ADCC activities of TT patients were comparable to those of healthy donors. It therefore appears that, like the T-cell functions, noninducible cellular effector mechanisms are also unimpaired in TT patients. However, NK cytotoxicity could not be modulated well in TT patients. Tung, et al. (19) reported reduced soluble IL-2 receptor levels in the sera of TT and BT patients. Whether the inability of lymphocytes from TT patients to optimally respond to lymphokincs can be attributed to their receptor status needs further investigation.
The role of the impaired NK and ADCC activities in the pathogenesis of lepromatous leprosy remains unclear. The recognition of infected cells by effectors may be an important step in their cytotoxic function. In virus-infected cells, it has been shown that integration of viral proteins in target-cell membranes provides a recognition structure for the cytotoxic effectors. It is postulated that similar membrane changes may be occurring in cells harboring mycobacteria (14). It is also thought that once the bacteria are released from their protective environment by cellular cytotoxic mechanisms, they become vulnerable to destruction by cytotoxic cells or humoral antibodies (13,14). Recently, lymphokine-activated killer (LAK) cells have been shown to effectively lyse rickettsiae-infected target cells and murine macrophages infected with intracellular pathogens (3,18). These studies, along with ours, indicate a promising im-munotherapeutic role for NK/LAK cells in controlling intracellular infections.
Acknowiedsment. The authors wish to acknowledge financial support by the Indian Council of Medical Research, New Delhi, India, under the project " Im-munoprophylaxis trials of ICRC anti-leprosy vaccine" and the European Economic Community, Brussels, under the project " Immunology and immunoprophylaxis of leprosy." We are grateful to Dr. K. K. Koticha, Superintendent, Acworth Leprosy Hospital, Bombay, for his help.
REFERENCES
1. Blanchard. D. K.. Stewart, W. E., Klein, T. W., Friedman, H. and Djeu, J. Y. Cytolytic activity of human peripheral blood leukocytes against Legionella pneumophilia-infected monocytes: characterization of the effector cell and augmentation by interleukin-2. J. Immunol. 139(1987)551-556.
2. Bloom. B. R. and Mehra. V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80(1984)5-28.
3. Carl, M and DascH, G. A. Characterization of human cytotoxic lymphocytes directed against cells infected with typhus group rickettsiae: evidence for lymphokine activation of effectors. J. Immunol. 136(1986)2654-2661.
4. Chai, S. R. and Lee, C. H. A study of natural killer cell activity from the peripheral blood of leprosy patients. (Abstract) Int. J. Lepr. 52Suppl.(1984)696.
5. CHing. C. Y.. Pollack. M., Reichert, E., Hoka-ma, Y., Fujikawa. R.. Loui, W., Sato, ID., Wong, C. and Ching, N. Analysis of immunologic and genetic factors in multicase families with Hansen's disease in Hawaii. Int. J. Lepr. 53(1985)163-165.
6. Converse, P. J. and Bjune. G. Natural killer(NK) cell activity and reversal reaction in leprosy. Int. J. Lepr. 54(1986)503-509.
7. Dahholkar. M., Advani. S., Tatake. R. and Gangal, S. Natural and antibody-dependent cellular cytotoxicity in chronic myeloid leukemia patients in remission. Leukemia Res. 10(1986)203-209.
8. Garcia-PenarruBia, P., Bankhurst, A. D. and Koster, F. T. Experimental and theoretical kinetics study of anti-bacterial killing mediated by human natural killer cells. J. Immunol. 142(1989)1310-1317.
9. Hanna, N. The role of natural killer cells in the control of tumorgrowth and metastasis. Biochimi-ca Biophys. Acta 780(1985)213-226.
10. Hersh, E. V.. Murphy, S. G., Gutterman, J. U., Morgan, J., Quesada, J., Zander, A. and Stewart, D. Antibody-dependent cell-mediated cytotoxicity in human cancer: characterization of patient leukocyte activity and treatment effects. Cancer 49(1982)251-260.
11. Humphres, R. C, Gelber, R. H. and Krahen-buhl. J. L. Suppressed natural killer cell activity during episodes of erythema nodosum leprosum in Iepromatous leprosy. Clin. Exp. Immunol. 49(1982)500-508.
12. Kaplan, G.. Laal, S., Sheftel, G., Nusrat, A., Nath, I.. MatHur, N. K., Mishra. R. S. and Cohn, Z. A. The nature and kinetics of a delayed immune response to purified protein derivative of tuberculin in the skin of lepromatous leprosy patients. J. Exp. Med. 168(1988)1811-1824.
13. Kaufmann, S. H. E. CD8+ T lymphocytes in intracellular microbial infections. Immunol. Today 9(1988)168-174.
14. Klimpel, G. R., Niesel. D. W. and Klimpel, K. D. Natural cytotoxic effector cell activity against Shigella flexneri infected HeLa cells. J. Immunol. 136(1986)1081-1086.
15. Lee, K. H.. Haw. C. R. and Lim, S. K. In vitro effect of human alpha interferon on natural killer (NK) cell activity in patients with lepromatous leprosy. (Abstract) Int. J. Lepr. 54(1986)345.
16. Martz, E. and Howell. D. M. CTL: virus control cells, first and cytolytic cells second? Immunol. Today 10(1989)79-86.
17. Ortaldo, J. R. Regulation of natural killer activity. Cancer Metastasis Rev. 6(1987)637-651.
18. Resnick, M.. Roguel. N., Bercovier, H., Enk, C, Frankenburg, S. and Kedar, E. Lysis of murine macrophages infected with intracellular pathogens by interleukin-2 activated killer (LAK) cells in vitro. Cell. Immunol. 113(1988)214-219.
19. Tung, K. S., Umland. E., Matzner, P., Nelson, E.. Schauf, V., Rubin, L., Wagner. D., Scollard, D., VITHAYASAI. P., VITHAYASAI, V., worobec, S., Smith, T. and Suriyanond, V. Soluble serum interleukin-2 receptor levels in leprosy patients. Clin. Exp. Immunol. 69(1987)10-15.
20. Welsh, R. M. Regulation of virus infections by natural killer cells: a review. Nat. Immun. Cell Growth Regul. 5(1986)169-199.
1. Ph.D.; Cancer Research Institute, Tata Memorial Centre, Parel, Bombay 400012. India.
2. B.Sc; Cancer Research Institute, Tata Memorial Centre, Parel, Bombay 400012. India.
3. M.D.. Ph.D., Cancer Research Institute, Tata Memorial Centre, Parel, Bombay 400012. India.
4. Ph.D., Cancer Research Institute, Tata Memorial Centre, Parel, Bombay 400012. India.
5. M.B.B.S., M.P.H., Richardson Leprosy Hospital, Miraj, India.
6. M.B.Bch., Richardson Leprosy Hospital, Miraj, India.
7. M.B.B.S., D.V.D., D.P.H. Acworth Leprosy Hospital, Bombay, India.
8. R. G. Chulawalla, B.Sc, Acworth Leprosy Hospital, Bombay, India.
Reprint requests to Dr. Mrs. Sudha G. Gangal, Head, Immunology Division, Cancer Research Institute, Tata Memorial Centre, Parel, Bombay 400012, India.
Received for publication on 7 August 1989.
Accepted for publication in revised form on 13 December 1989.