- Volume 58 , Number 2
- Page: 342–6
Effect of Mycobacterium leprae's phenolic glycolipid-I on interferon-gamma augmentation of monocyte oxidative responses
ABSTRACT
Peripheral blood monocytes were pretreated with phenolic glycolipid-I (PGL-I), dimycocerosyl phthioccrol (DIM), or mycoside A, then cultured in the presence or absence of interferon-gamma (IFN-γ).Their oxidative responses to Mycobacterium leprae, phorbol myristate acetate (PMA), and opsonized zymosan were evaluated. In response to M. leprae, monocytes pretreated with PGL-I released less O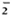 than nonlipid-treated control cells. The IFN-γ augmentation of oxidative responses was suppressed only in PGL-I-pretreated monocytes and only when the stimulus was M. leprae. This suggests that PGL-I, by affecting the IFN-γ enhancement of phagocytic cell oxidative responses, aids further the intracellular survival of M. leprae.
than nonlipid-treated control cells. The IFN-γ augmentation of oxidative responses was suppressed only in PGL-I-pretreated monocytes and only when the stimulus was M. leprae. This suggests that PGL-I, by affecting the IFN-γ enhancement of phagocytic cell oxidative responses, aids further the intracellular survival of M. leprae. RÉSUMÉ
Après avoir été prétraités par l'antigène phénogly-colipidique-1 (PGL-1), le dimycocerosyl phthiocerol (DIM), our le mycoside A, des monocytes du sang périphérique ont été cultivés en présence ou en absence d'interferon-gamma (IFN-γ). On a alors évalué leur réponse oxydative au Mycobacterium leprae, au my-ristate acétate de phorbol (PMA), et au zymosan opso-nisé. En présence de M. leprae, les monocytes prétraités avec le PGL-I ont libéré moins d'oxygène que les cellules témoins non traitées par des lipides. L'augmentation de la réponse oxydative en présence d'IFN-γ a été supprimée uniquement dans les monocytes prétraités par le PGL-I, mais ceci seulement lorsqu'ils étaient stimulées par M. leprae. Ces observations suggèrent que le PGL-I, en entraînant un renforcement de la réponse oxydative des cellules phagocytaires en présence d'IFN-γ, contribue à assurer la survie intracellulaire de M. leprae.RESUMEN
Se pretrataron monocitos de sangre periférica con glicolipido fenólico-1 (PGL-1), con dimicocerosil phtiocerol (DIM), o con micósido A y después se cultivaron en presencia o ausencia de interferon gamma (IFN-γ). Enseguida se evaluaron sus respuestas oxidatives hacia el Mycobacterium leprae, hacia el acetato de forbol miristato (PMA) y hacia levaduras opsoni-zadas. Los monocitos pretratados con PGL-1 liberaron menos O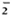 en respuesta al M. leprae que los controles no tratados con el lípido. El aumento de la respuesta oxidativa inducido por el IFN-γ, lue suprimido solo en los monocitos pretratados con PGL-1 y solo cuando el estimulo fue M. leprae. Esto sugiere que el PGL-1, al afectar el incremento en la respuesta oxidativa de la célula fagocitica inducido por el IFN-γ, ayuda a la supervivencia intracelular del M. leprae.
en respuesta al M. leprae que los controles no tratados con el lípido. El aumento de la respuesta oxidativa inducido por el IFN-γ, lue suprimido solo en los monocitos pretratados con PGL-1 y solo cuando el estimulo fue M. leprae. Esto sugiere que el PGL-1, al afectar el incremento en la respuesta oxidativa de la célula fagocitica inducido por el IFN-γ, ayuda a la supervivencia intracelular del M. leprae. Mycobacterium leprae synthesizes a unique phenolic glycolipid (PGL-I). It is a phenol-phthiocerol triglyceride with an antigenically distinct trisaccharide, 3,6-di-O-methyl-β-D-glucopyranosyl-(l→4)-2,3-di-O-methyl-α-L-rhamnopyranosyl-(1→2)-2,3-di-O-methyl-α-L-rhamnopyranose that constitutes 2% of the mass of isolated M. leprae (3). It has been detected in the sera of leprosy patients (1,17) as well as in skin biopsies of lepromatous leprosy patients (15,16). PGL-I is structurally similar to my-coside A of M. kansasii, differing in the trisaccharide moiety. PGL-I and the related non-glycosylated, non-phenylated dimyco-ccrosyl phthiocerol (DIM) are found in large amounts in the tissues of experimentally infected armadillos (3,4). M. leprae releases the lipids into its milieu since the lipids are found in tissues freed of organisms.
The specific immunological unresponsiveness seen in lepromatous leprosy patients may be due in part to the accumulation of large amounts of PGL-I in tissues infected with M. leprae. It has been reported that PGL-I suppresses mitogenic responses of lymphocytes from lepromatous leprosy patients (7). In addition, these patients have been shown to have defective interferon-gamma (IFN-γ)production (9). Mononuclear phagocytes have been shown to serve as host cells for M. leprae. We have previously shown (14) that peripheral blood monocytes pretreated with PGL-I release less superoxide anion (O2) when stimulated with M. leprae than do control monocytes. On the other hand, monocytes pretreated with dimycocerosyl phthiocerol (DIM), mycoside A of M. kansasii, or mycoside B of M. microti, release O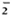 in quantities comparable to control monocytes in response to M. leprae stimulation. Monocyte O
in quantities comparable to control monocytes in response to M. leprae stimulation. Monocyte O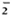 release in response to other stimuli of the oxidative metabolic burst, such as phorbol myristatc acetate (PMA), zymosan, M. bovis BCG, or M. kansasii, was unaffected by lipid pretreatment. These data demonstrate that PGL-I affects monocyte O
release in response to other stimuli of the oxidative metabolic burst, such as phorbol myristatc acetate (PMA), zymosan, M. bovis BCG, or M. kansasii, was unaffected by lipid pretreatment. These data demonstrate that PGL-I affects monocyte O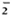 generation specifically when the challenging organism is M. leprae, and suggest that PGL-I plays an important role in the pathogenesis of leprosy. We undertook this study to determine if PGL-I affects the IFN-γactivation of monocytes and thus contributes further to the intracellular survival of M. leprae.
generation specifically when the challenging organism is M. leprae, and suggest that PGL-I plays an important role in the pathogenesis of leprosy. We undertook this study to determine if PGL-I affects the IFN-γactivation of monocytes and thus contributes further to the intracellular survival of M. leprae.
MATERIALS AND METHODS
Lipids. The lipids used in this study, PGL-I, DIM, and mycoside A, were kindly supplied by Dr. Patrick J. Brennan (Colorado State University, Fort Collins, Colorado, U.S.A.) through National Institutes of Health contract no. AI-52582. The lipids were purified as described previously (3,4). To assure stability, lipid sonicates were prepared immediately prior to monocyte exposure as previously outlined (14).
Cells. Peripheral blood was drawn from consenting healthy volunteers into heparinized (10 IU/ml) syringes. Monocytes were isolated as described (14). Briefly, mononuclear cells were obtained following centrifugation of the diluted blood over Ficoll-Hypaque (Sigma Chemical Co., St. Louis, Missouri, U.S.A. and Winthrop-Breon, New York, New York, U.S.A.). The monocytes were then obtained by elutriation using a Beckman model J2-21 centrifuge with a JE-6B rotor. A flow rate of 21 ml/min and rotor speed of 600 xg were used to exclude the lymphocytes, contaminating platelets, and erythrocytes. The flow rate was then increased to 28 ml/min and 200 ml of the clutriation buffer was collected to obtain the monocytes. After washing, the cells were resuspended in RPMI 1640 (Whittaker Bio-products, Inc., Walkersville, Maryland, U.S.A.) medium containing 10% heat-inactivated fetal bovine serum (Hyclone Laboratories, Inc., Logan, Utah, U.S.A.) and 50 μg/ml gentamicin, and then counted. Viability was always greater than 98% as determined by trypan blue dye exclusion. Latex ingestion was used to determine the percentage of phagocytic monocytes (2), and this was consistently greater than 85%.
Culture conditions. Sonicates of either PGL-I, DIM, mycoside A, or buffer alone were added to the monocytes in Teflon vials (Savillex, Minnetonka, Minnesota, U.S.A.) at 10 μg lipid/2 x106 cells. The vials were tumbled end over end at 12 rotations/min for 2 hr at 37ºC. We have previously determined that under these conditions an average of 15 μg PGL-I/2 x106 cells was taken up by the monocytes as determined by dot-blot analysis following the extraction of the PGL-I from the monocytes (14). We have also shown that comparable amounts of DIM and mycoside A were taken up by the monocytes, and that the cells were viable after treatment with the various lipids (14). The cells were washed by centrifugation and then incubated in 96-well, flat-bottom microliter plates (Corning, Inc., Corning, New York, U.S.A.) at 2 x105 cells/well in a 0.2 ml volume of medium for 2 hr at 37ºC in 5% CO2 in humidified air to allow adherence. After washing, the cells were incubated overnight in 0.2 ml/well of medium alone or medium containing 500 U/ml IFN-γ (purified native human IFN-γ ;Sigma) at 37ºC in 5% CO2 in humidified air.
Superoxide anion assay. After washing the wells, O release by the adherent monocytes was determined by measuring the superoxide dismutase (SOD)-inhibitable cytochrome C (Sigma) reduction as described previously (2,10,14). The stimuli used in the assay included PMA (500 ng/ml; Sigma), opsonized zymosan (1:1 v/v with fresh serum at 37ºC for 20 min), and killed M. leprae (50:1 particlc-to-monocytc ratio). The preparation of the bacteria for use in the assay has been described (2). The amount of cell protein, as determined by the Lowry method (6), did not differ significantly between the experimental and control groups.
release by the adherent monocytes was determined by measuring the superoxide dismutase (SOD)-inhibitable cytochrome C (Sigma) reduction as described previously (2,10,14). The stimuli used in the assay included PMA (500 ng/ml; Sigma), opsonized zymosan (1:1 v/v with fresh serum at 37ºC for 20 min), and killed M. leprae (50:1 particlc-to-monocytc ratio). The preparation of the bacteria for use in the assay has been described (2). The amount of cell protein, as determined by the Lowry method (6), did not differ significantly between the experimental and control groups.
Endotoxin contamination. Endotoxin was not detected in the buffers, medium, or lipid preparations used to treat monocytes as determined by a quantitative chromogenic limulus amebocyte lysate assay (Whittaker) with a sensitivity of 10 pg/ml.
Statistical analysis. The differences between control and experimental groups were evaluated for statistical significance by Student's t test.
RESULTS
The O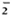 release by untreated control cells was about twice that of PGL-I-pretreated monocytes when M. leprae was used as the stimulus (Fig. 1A). Pretrcatmcnt with mycoside A of M. kansasii or the non-glyco-sylated non-phenylated dimycocerosyl phthiocerol (DIM) did not significantly alter the O
release by untreated control cells was about twice that of PGL-I-pretreated monocytes when M. leprae was used as the stimulus (Fig. 1A). Pretrcatmcnt with mycoside A of M. kansasii or the non-glyco-sylated non-phenylated dimycocerosyl phthiocerol (DIM) did not significantly alter the O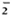 release in response to M. leprae. IFN-γ augmented the release by all cells regardless of lipid pretrcatmcnt. However, the O
release in response to M. leprae. IFN-γ augmented the release by all cells regardless of lipid pretrcatmcnt. However, the O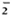 release by PGL-I-pretreated monocytes when activated by IFN-γ was significantly less than that of untreated monocytes activated by IFN-γ . IFN-γ was only able to enhance the O
release by PGL-I-pretreated monocytes when activated by IFN-γ was significantly less than that of untreated monocytes activated by IFN-γ . IFN-γ was only able to enhance the O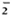 release of the PGL-I-treated cells to the same level as the preactivated state of the untreated monocytes. When the monocytes were pretreated with PGL-I, DIM, mycoside A, or buffer alone and then stimulated with PMA (Fig. 1B) or with opsonized zymosan (Fig. 1C), they released comparable amounts of O
release of the PGL-I-treated cells to the same level as the preactivated state of the untreated monocytes. When the monocytes were pretreated with PGL-I, DIM, mycoside A, or buffer alone and then stimulated with PMA (Fig. 1B) or with opsonized zymosan (Fig. 1C), they released comparable amounts of O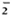 . IFN-γ was able to augment the O
. IFN-γ was able to augment the O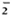 generation by all of the cell groups equally well.
generation by all of the cell groups equally well.
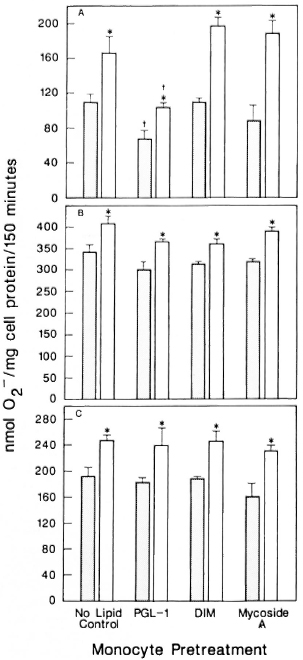
The Figure. Effect of mycobacterial lipids on IFN-γaugmentation of monocyte release. Monocytes were pretreated with indicated lipids and then cultured in the presence ( ) or absence (
) or absence ( ) of IFN-γ overnight. The cells were then stimulated with (A) M. leprae, (B) PMA, or (C) opsonized zymosan, and O
) of IFN-γ overnight. The cells were then stimulated with (A) M. leprae, (B) PMA, or (C) opsonized zymosan, and O release measured. Results are means ± S.E. of 8 experiments in A, 6 experiments in B, and 4 experiments in C. * = p < 0.05 by Student's t test when compared with no IFN-γ treatment; † = P < 0.05 by Student's t test when compared with no lipid control pretreatment.
release measured. Results are means ± S.E. of 8 experiments in A, 6 experiments in B, and 4 experiments in C. * = p < 0.05 by Student's t test when compared with no IFN-γ treatment; † = P < 0.05 by Student's t test when compared with no lipid control pretreatment.
DISCUSSION
Our data indicate that, even though PGL-I suppresses O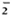 release by monocytes stimulated with M. leprae, these cells can be activated by IFN-γ. However, the degree of activation was significantly less than that observed with control monocytes. It has been shown by us and by others (7,14) that PGL-I can suppress T cell and monocyte function. It has also been reported that IFN-γ production is decreased in lepro-matous leprosy patients (9), but that monocytes from those patients can respond to IFN-γ with augmented oxygen radical production (5). However, in studies using mouse macrophages, it was shown that foot pad macrophages gorged with M. leprae are refractory to activation by IFN-γ (12), and peritoneal macrophages infected with M. leprae respond to IFN-γ if it is administered within 24 hr of infection but not if administered 3 to 5 days postinfection (13). In our studies with human monocytes, we attempted to use experimental conditions that resemble those found in vivo in lepromatous leprosy (14), where the cells are exposed to products of M. leprae, such as PGL-I. We have reported that the various lipids were taken up by the monocytes in comparable amounts (14). We have shown that PGL-I-pretreated monocytes released less O
release by monocytes stimulated with M. leprae, these cells can be activated by IFN-γ. However, the degree of activation was significantly less than that observed with control monocytes. It has been shown by us and by others (7,14) that PGL-I can suppress T cell and monocyte function. It has also been reported that IFN-γ production is decreased in lepro-matous leprosy patients (9), but that monocytes from those patients can respond to IFN-γ with augmented oxygen radical production (5). However, in studies using mouse macrophages, it was shown that foot pad macrophages gorged with M. leprae are refractory to activation by IFN-γ (12), and peritoneal macrophages infected with M. leprae respond to IFN-γ if it is administered within 24 hr of infection but not if administered 3 to 5 days postinfection (13). In our studies with human monocytes, we attempted to use experimental conditions that resemble those found in vivo in lepromatous leprosy (14), where the cells are exposed to products of M. leprae, such as PGL-I. We have reported that the various lipids were taken up by the monocytes in comparable amounts (14). We have shown that PGL-I-pretreated monocytes released less O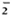 , only when stimulated with M. leprae, than did control cells or cells pretreated with lipids structurally similar to PGL-I. Here, wc have also shown that cells pretreated with PGL-I respond to IFN-γ with an increased oxidative potential but to a lesser degree than control cells when the stimulus is M. leprae. PGL-I may contribute further to the intracellular survival of M. leprae by down-regulating the IFN-γenhancement of monocyte/macrophage oxidative responses. In ongoing clinical trials using intradermal administration of IFN-γ(8,11), the suppressive effects of accumulated PGL-I in the macrophages of leprosy lesions should be considered.
, only when stimulated with M. leprae, than did control cells or cells pretreated with lipids structurally similar to PGL-I. Here, wc have also shown that cells pretreated with PGL-I respond to IFN-γ with an increased oxidative potential but to a lesser degree than control cells when the stimulus is M. leprae. PGL-I may contribute further to the intracellular survival of M. leprae by down-regulating the IFN-γenhancement of monocyte/macrophage oxidative responses. In ongoing clinical trials using intradermal administration of IFN-γ(8,11), the suppressive effects of accumulated PGL-I in the macrophages of leprosy lesions should be considered.
Acknowledgments. This work was supported by the University of Illinois Earl M. Bane Trust Fund, the Veterans Administration Merit Review Grant MRIS-034, and the National Institutes of Health Grant AI-21354. We thank Dr. Patrick J. Brennan for supplying M. leprae, PGL-I, DIM, and mycoside A. We are grateful to Bea Wozniak for skillful secretarial assistance.
REFERENCES
1. Cho, S., Hunter, S. W., Gelber, R. H., Rea, T. H. and Brennan, P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153(1986)560-569.
2. Holzer, T. J., KizLAiTis, L., Vachula, M., Weaver, C. W. and Andersen, B. R. Human phagocytic cell responses to Mycobacterium leprae and Mycobacterium bovis bacillus Calmette Guerin: an in vitro comparison of leprosy vaccine components. J. Immunol. 141(1988)1701-1708.
3. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacterid. 147(1981)728-735.
4. Hunter, S. W. and Brennan, P. J. Further specific extracellular phenolic glycolipid antigens and a related diacylphthiocerol from Mycobacterium leprae. J. Biol. Chem. 258(1983)7556-7562.
5. Kaplan, G., Nathan, C. F., Gandhi, R., Hor-witz, M. A.. Levis, W. R. and Cohn, Z. A. Effect of recombinant interferon-γ on hydrogen peroxide-releasing capacity of monocyte-derived macrophages from patients with lepromatous leprosy. J. Immunol. 137(1986)983-987.
6. Lowry, O. H., Rosehrough, N. J., Farr, A. L. and Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1951)265-275.
7. Meura. V., Brennan, P. J., Rada, E., Convit, J. and Bloom, B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308(1984)194-196.
8. Nathan, C. F.. Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M. D., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
9. Nogueira, N., Kaplan, G., Levy, E., Sarno, E. N., Kushner, P., Granelli-Piperno, A., Vieira, L., Gould. V. C, Levis, W., Steinman, R„ Yip, Y. K. and Cohn. Z. A. Defective 7-interferon production in leprosy. J. Exper. Med. 158(1983)2165-2170.
10. Pick. E. and Mizel, D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Methods 46(1981)211-226.
11. Samuel, N. M., Grange, J. M., Samuel, S., Lucas, S., Owilli, O. M., Adalla, S., Leigh, I. M. and Navarrette, C. A study of the effects of intradermal administration of recombinant gamma interferon in lepromatous leprosy patients. Lepr. Rev. 58(1987)389-100.
12. Sibley, L. D. and Krahenbuhl, J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect. Immun. 55(1987)446-450.
13. Sibley, L. D. and Krahenbuhl, J. L. Induction of unresponsiveness to gamma interferon in macrophages infected with Mycobacterium leprae. Infect. Immun. 56(1988)1912-1919.
14. Vachula, M., Holzer, T. J. and Andersen, B. R. Suppression of monocyte oxidative responses by phenolic glycolipid I of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.
15. Vemuri, N., Khandke, L., Maiiadevan, P. R., Hunter. S. W. and Brennan, P. J. Isolation of phenolic glycolipid I from human lepromatous nodules. Letter. Int. J. Lepr. 53(1985)487-489.
16. Young, D. B. Detection of mycobacterial lipids in skin biopsies from leprosy patients. Int. J. Lepr. 49(1981)198-202.
17. Young, D. B., Harnisch, J. P., Knight, J. and Buchanan, T. M. Detection of phenolic glycolipid I in sera from patients with lepromatous leprosy. J. Infect. Dis. 152(1985)1078-1081.
1. Ph.D., Departments of Medicine and Microbiology/Immunology, University of Illinois at Chicago, Chicago, Illinois.
2. Ph.D., Department of Microbiology/Immunology, University of Illinois at Chicago and West Side Veterans Administration Medical Center, Chicago, Illinois.
3. U.S., West Side Veterans Administration Medical Center, Chicago, Illinois.
4. M.D., Departments of Medicine and Microbiology/Immunology, University of Illinois at Chicago, and West Side Veterans Administration Medical Center. Chicago, Illinois, U.S.A.
Present addresses: M. Vachula, Ph.D., Baxter Healthcare, WG2-2S, Route 120 and Wilson Road, Round Lake, Illinois 60073, U.S.A. T. J. Holzer, Ph.D., Abbott Laboratories, 1400 Sheridan Road, North Chicago, Illinois 60064, U.S.A.
Reprint requests to: Dr. Burton R. Andersen, Chief, Section of Infectious Diseases, M/P 151, West Side VA Medical Center, 820 S. Damen Avenue, Chicago. Illinois 60612. U.S.A.
Received for publication on 14 August 1989.
Accepted for publication in revised form on 16 January 1990.