- Volume 58 , Number 2
- Page: 353–7
Acid-fast bacilli found in sphagnum vegetation of coastal norway containing Mycobacterium leprae-specific Phenolic glycolipid-I
ABSTRACT
In the grey layer of sphagnum vegetation originating f rom former leprosy-endemic regions of coastal Norway, acid-fast bacilli (AFB) containing Mycobacterium leprae-specific phenolic glycolipid I (PGL-I) on the surface have been found. These AFB survived in foot pads of nude mice with multiplication but without swelling. This contrasts to experimental leprosy with clinically derived M. leprae where swelling and unlimited multiplication takes place. The naturally occurring AFB may be of a lower pathogenicity than M. leprae obtained f rom clinical cases. The possibility of M. leprae surviving in sphagnum vegetation was assessed by inoculation of clinically derived M. leprae into the grey layer of the sphagnum. It multiplied more than tenfold and retained its pathogenicity in nude mice for 16 weeks, the duration of the experiment. The lack of pathogenicity of sphagnum-derived, M. leprae-like mycobacteria may be relevant to the decline of leprosy in Norway.RÉSUMÉ
On a décelé des bacilles acido-résistants (AFB) contenant l'antigène phénoglycolipidique-I (PGL-I) spécifique pour Mycobacteriitm leprae, à la surface de la couche grise d'une végétation de spheignes provenant de régions auparavant endémiques pour la lèpre dans la région côtière de la Norvège. Ces bacilles acido-résistants ont survécu dans les coussinets plantaires de souris nues, en se multipliant, mais sans témoigner de gonflement. Ces observations ne correspondent pas à ce qu'on observe dans la lèpre expérimentale avec M. leprae obtenus chez des malades, où le gonflement et une multiplication illimités prennent place. Les bacilles acido-résistants naturels pourraient témoigner d'une pathogénicité plus faible que M. leprae recueilli à partir de lésions cliniques. La possibilité d'une survie de M. leprae dans la végétation de spheignes a été évaluée par l'inoculation au niveau des couches grises des spheignes de bacilles de la lèpre obtenus chez des malades cliniques. Ces bacilles se sont multipliés plus de 10 fois et ont gardé leur pathogénicité chez la souris nue pendant 16 semaines, ce qui correspond à la durée de l'expérience. L'absence de pathogénicité des my-cobactéries ressemblant à M. leprae et obtenues dans les spheignes pourrait avoir joué un rôle dans le déclin de la lèpre en Norvège.RESUMEN
Se han encontrado bacilos ácido-resistentes (BAAR) conteniendo en su superficie al glicolipido fenólico-1 específico del Mycobacterium leprae en la capa gris de la vegetación musgosa encontrada en las regiones anteriormente endémicas en lepra de las costas de Noruega. Estos BAAR sobrevivieron en las almohadillas plantares del ratón desnudo, con multiplicación pero sin linchamiento. Esto contrasta con la lepra experimental causada por el M. leprae derivado de casos clínicos, donde ocurre hinchamiento y multiplicación ilimitada. Los BAAR encontrados en el musgo pueden tener una menor patogenicidad que los M. leprae obtenidos de casos clínicos. La posibilidad de que M. leprae sobreviviera en el musgo se analizó inoculando M. leprae de casos clínicos en la capa gris del musgo. Aquí, el M. leprae se multiplicó mas de 10 veces y retuvo su patogenicidad en el ratón desnudo durante 16 semanas, la duración del experimento. La falta de patogenicidad de la micobacteria derivada del musgo, podría ser relevante en la disminución de la lepra en Noruega.The existence of noncultivable acid-fast bacilli (AFB) in sphagnum moss vegetation of former leprosy regions in coastal Norway has previously been reported (6). After the inoculation of these bacilli into nine-banded armadillos, antibodies reacting with Mycobacterium leprae-specific antigen 7 (as tested by Prof. Harboe, Oslo5) were identified, strongly suggesting that these mycobacteria were M. leprae.
In an attempt to further identify these mycobacteria, additional samples of sphagnum vegetation were collected in previously leprosy-endemic areas of western Norway. The AFB in these samples were examined with monoclonal antibodies against the phenolic glycolipid-1 (PGL-I) of M. leprae (7) and in the nude mouse model. In addition, to assess the possibilities of M. leprae propagating in such biotopes, M. leprae was inoculated into samples of this sphagnum vegetation and its survival was monitored over a period of time.
MATERIALS AND METHODS
In Naustdal Parish of Sunnfjord, Norway (61º30'N, 5º30'E), five sphagnum biotopes were selected according to criteria established by Irgens, et al. (4) for the selection of sphagnum biotopes in which a high concentration of AFB might be expected. Each sample consisted of all the grey layer from an area of about 1 square meter of well-growing Sphagnum rubellum, S. magellanicum, and S. imbricatum. The samples were handled with sterile plastic gloves, and transported within 14 days to the laboratory where the material was stored at + 4ºC until processed.
From the five biotopes, a total of nine samples was collected in the beginning of July 1986. Sterile plastic syringes were filled with approximately 100 cc of the grey layer, compressed, and the fluid collected. The compressed vegetation was weighed for the calculation of AFB count/g. For further processing, the fluid was centrifuged at 8000 x g x 30 min, and the sediment diluted in 2 ml of M/30 phosphate buffer, pH 7.0.
The first examination of the sediment took place within 4 weeks after the collection; the second, after the vegetation had been incubated for a period of 2 months at 34ºC and 22ºC in 12-hr pulses to simulate conditions optimal for the growth of noncultivablc AFB (6). In both examinations, the number of AFB were counted. In the second examination, the AFB showing a positive reaction with M. leprae-specific monoclonal antibodies (MAbs) were counted separately. In this reaction, an indirect immunofluorescence technique (IFT) was used as previously described (7).
In the IFT procedure 10 spots were used on each slide, in duplicate, for each experiment. The control was coated with a suspension of armadillo-derived M. leprae (spots 1 and 2). The negative control contained M. avium serotype 2 (spots 3 and 4). Spots 5, 6, 7, and 8 were used for the experiments. They were coated with the suspensions to be examined. The last two spots served as further negative controls coated with M. leprae (spot 9) or M. avium serotype 2 (spot 10) but without any MAbs.
In the experiments, the M. leprae-specific MAb F47-21 against the PGL-I was used for spots 1,3,5,6, and 7. In addition, MAb F85-2, specific for M. avium serotype 2, was used in spots 2, 4, and 8. For the evaluation of PGL-I-positive bacilli, a pronounced immunofluorescence in spots 5, 6, and 7 together with a characteristic rod-shaped appearance was considered. The remaining spots were negative except for spot 1 (positive control for the known M. leprae suspension) and spot 4 (positive control for M. avium). Furthermore, 0.03 ml of the diluted sediment was injected into the right hind food pads of nude mice (CD-I, nu/nu SPF) using five animals for each sample. At 1-month intervals, the swelling of the foot pads was measured and compared with the noninoculated left hind foot pads. Six, 8, and 9 months after inoculation, 1-2 animals were autopsied, and the inoculated foot pad was homogenized (6) and examined for the presence of AFB in IFT with MAb 47-21 of living cells (8) and of cultivable mycobacteria (Löwenstein-Jensen and Middle-brook 7H10 solid media). In three instances, IFT-positive homogenates were inoculated into other nude mice.
To assess the potential of M. leprae to survive and multiply in the grey layer of the sphagnum, a sample from biotope 1 was chosen. Altogether, five series of examinations were performed in which 20-ml sterile plastic syringes were filled with sphagnum. In the first series, the syringes were inoculated with armadillo-derived M. leprae (1) from the liver of nine-banded armadillo no. 115 (originating from the Leprosy Tissue Bank, Research Institute, Borstel) suspended in 2 ml of sterile-filtered sphagnum fluid in a concentration of 5.9 x 107 M . leprae/cc of sphagnum. In the second series, the syringes were inoculated with M. leprae at a concentration of 5.9 x 106 M. leprae/cc sphagnum. Viability of the M. leprae cells was tested using the fluorescein diacetate (FDA) and ethidium bromide (EB) reactions (8). The third series was not inoculated and served as a control. In the fourth and fifth scries, the sphagnum was heated for 30 min at 121ºC and then inoculated as in the first and second series. From each series, two syringes were examined at 1,2, 5, 6, 10, 12, and 16 weeks after inoculation.
RESULTS
In all nine sphagnum samples, AFB were found (Table 1). During incubation, the average AFB count increased from 2.35 x 105 to 3.15 x 106 of compressed vegetation. After incubation, PGL-I-positive AFB were found in all samples with morphology and fluorescence similar to that of M. leprae (The Figure).
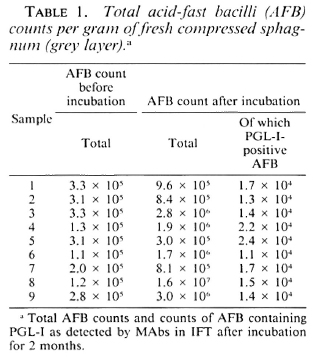
The Figure. Bacilli in sphagnum moss vegetation show a marked fluorescence (→) when PGL-I-specific monoclonal antibodies are used (indirect immunofluorescence technique, MAb 47-21-3).
When inoculated into nude mice and examined over 9 months, no foot-pad swelling was observed. However, the concentration of PGL-I-positive AFB increased in 3 samples, was stable in 2 samples, and decreased in 2 samples. In 2 out of the 9 samples, no PGL-I-positive AFB were found after 6 months. FDA-positive AFB, implying viable cells, were found at one or more examinations in all samples, except for the two samples without PGL-I-positive AFB after 6 months. In three of the samples, cultivable mycobacteria were present 6 months after inoculation; however, after 9 months no cultivable mycobacteria were found.
After inoculation into the grey layer of sphagnum vegetation, the count of M. leprae diminished during the first week (Table 2). During the next 15 weeks, a more than tenfold increase was observed which was more or less independent of the number of M. leprae inoculated. After 16 weeks of incubation, when inoculated into nude mice these AFB produced swelling similar to the pattern of M. leprae. The count of PGL-I-positive AFB in noninoculated sphagnum vegetation remained about the same throughout the culture period. These PGL-I-positive AFB did not produce foot-pad swelling in nude mice. When inoculated into heated sphagnum, M. leprae disappeared within 6 weeks.
DISCUSSION
The present results confirm, by a more specific method, our previous findings (5,6) in sphagnum vegetation of viable microorganisms which cannot be differentiated from M. leprae. Certainly, the specificity of the method based on PGL-I may be questioned. However, until now, mycobacteria other than M. leprae have not been found to contain PGL-I on the cell surface, and the specificity of the monoclonal antibodies against PGL-I used in this study have been confirmed by a World Health Organization workshop (2). To assess the possibilities of M. leprae surviving in sphagnum vegetation, the growth of M. leprae was monitored in such vegetation over 16 weeks. It appeared that M. leprae incubated in non-heated vegetation multiplied more than tenfold and retained its pathogenicity for nude mice. Lack of foot-pad swelling in nude mice together with a limited multiplication suggest that these AFB may be of lower pathogenicity than the M. leprae obtained from clinical cases. Still, these AFB survive and to some extent multiply in nude mice. Whether a range in pathogenicity is a characteristic of M. leprae, perhaps also relevant to the differentiation in various clinical forms, needs further clarification.
The present findings add to the etiological role of environmental M. leprae. It has been found previously that in the health districts with the highest incidence rates of leprosy in Norway, a statistically significant association existed on a farm between the incidence of leprosy and the conditions relevant to the occurrence of mycobacteria in its environment (3). The gradual decline of leprosy in Norway certainly relates to a series of factors (3), but the lack of pathogenicity of naturally occurring organisms for nude mice found in the present study is consistent with no new cases of leprosy having been recorded in Norway since the 1950s. If this mechanism was responsible for the decline, antibodies against PGL-I might still be expected to exist in the Norwegian population. The demonstration of such antibodies would be evidence to support the hypothesis, and examinations aimed at the clarification of this issue should be pursued.
Acknowledgments. This study was financially supported by the German Leprosy Relief Association, Würzburg. We would like to thank Christian Irgens and Oliver Kazda for their help in collecting the sphagnum samples, and Mrs. Corina Dierfeld, Mr. Werner Mohr, and Mrs. Elke Link for their skillful technical assistance.
REFERENCES
1. Draper, P. Protocol 1/79 - purification of M. leprae. Report of the Enlarged Steering Committee for Research on the Immunology of Leprosy (IMMLEP) Meeting of 7-8 February 1979. Geneva: World Health Organization, 1979. Annex 1, p. 4.
2. Engers, et al. Results of a World Health Organization-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. (Letter) Infect. Immun. 48(1985)603-605.
3. Irgens, L. M. Leprosy in Norway; an epidemiological study based on a national patient registry. Lepr. Rev. 51Suppl.(1980)1-130.
4. Irgens, L. M., Kazda, J., MÜller, K. and Eide, G. E. Conditions relevant to the occurrence of acid-fast bacilli in sphagnum vegetation. Acta Pathol. Microbiol. Scand. [B] 89(1981)41-47.
5. Kazda, J. Occurrence of non-cultivable acid-fast bacilli in the environment and their relationship to M. leprae. Lepr. Rev. 52 Suppl. 1(1981)85-91.
6. Kazda, J., Irgens, L. M. and Müller, K. Isolation of non-cultivable acid-fast bacilli in sphagnum moss vegetation by foot pad technique in mice. Int. J. Lepr. 48(1980)1-6.
7. Kolk, A. H. J., Ho, M. L., Klatser, P. R., Eggelte, T. A. and Portaels, F. Production of monoclonal antibodies against Mycobacterium leprae and armadillo-derived mycobacteria. Ann. Inst. Pasteur Microbiol. 136B(1985)217-224.
8. Kvach, J. T., Munguia, G. and Strand, S. H. Staining tissue-derived Mycobacterium leprae with fluorescein diacetate and ethidium bromide. Int. J. Lepr. 52(1984)176-182.
1. M.V.D., Ph.D., Associate Professor, Institute for Experimental Biology and Medicine, Parkallec 22. D-2061 Borstel, Federal Republic of Germany.
2. M.D., Professor, Institute for Hygiene and Social Medicine, University of Bergen, N-5021 Bergen, Norway.
3. Ph.D., Royal Tropical Institute, 1105 AZ Amsterdam, The Netherlands.
Received for publication on 10 July 1989.
Accepted for publication in revised form on 23 October 1989.