- Volume 58 , Number 2
- Page: 358–64
Pathology of dual Mycobacterium leprae and simian immunodeficiency virus infection in rhesus monkeys
ABSTRACT
Three rhesus monkeys were experimentally inoculated with sooty-mangabey-derived Mycobacterium leprae and were inadvertently infected with the simian immunodeficiency virus (SIV) as well. They died of an immunodeficiency syndrome, and at autopsy all had lesions caused by M. leprae. One monkey was inoculated twice with M. leprae, initially with an inoculum f rom a sooty mangabey that was not infected with SIV and, subsequently, with an inoculum f rom a mangabey that was SIV infected. The monkey did not develop clinical lesions and became strongly lepromin skin test (LST) positive after the first inoculation, but became infected with both agents and LST negative following the second inoculation. These observations suggest that SIV-infected rhesus monkeys have an increased susceptibility to M. leprae infection and, by analogy, imply that HIV-infected human beings may have an increased susceptibility as well.RÉSUMÉ
Trois chimpanzés ont été inoculés expérimentalement par des bacilles de la lèpre obtenus à partir de singes mangabey; ces primates ont été également infectés par erreur avec une souche du virus du syndrome d'immuno-déficience simienne (VIS). Ces animaux ont développé un syndrome fatal d'immuno-déticience; l'autopsie a montré que toutes les lésions étaient causées par Mycobacterium leprae. Un des ces chimpanzés a été inoculé par deux fois avec M. leprae, tout d'abord avec un inoculât provenant d'un singe mangabey non infecté par SIV, et ensuite, par un inoculât provenant d'un singe mangabey qui était lui infecté par VIS. Ce chimpanzé n'a développé aucune lésion clinique; il est devenu fortement réactif a l'épreuve cutanée par la lépromine à la suite de la première inoculation, mais cette réaction est devenue négative à la suite de la seconde inoculation, lorsqu'il s'est vu infecté par les deux agents pathogènes. Ces observations suggèrent que les chimpanzés infectés par le VIS voient leur susceptibilité à l'infection par M. leprae accrue, ce qui par analogie implique que les humains infectés par VIH pourraient également témoigner d'une susceptibilité accrue.RESUMEN
Se inocularon 3 monos rhesus con Mycobacterium leprae obtenidos de un mono mangabey pardo y se infectaron inadvertidamente también con el virus de la inmunodeficiencia de los simios (SIV). Todos los monos murieron de un síndrome de inmunodeficiencia y en la autopsia todos tuvieron lesiones causadas por el M. leprae. Un mono se inoculó 2 veces con M. leprae; inicialmente con un inoculo derivado de un mono mangabey que no estaba infectado con SIV y subsecuentemente con un inoculo de un mono mangabey que sí lo estaba. El mono no desarrolló lesiones clínicas y resultó lepromino-positivo después de la primer inoculación pero se infectó con ambos agentes y se tornó lepromino-negativo después de la segunda inoculación. Estas observaciones sugieren que los monos rhesus infectados con el SIV tienen una susceptibilidad incrementada a la infección con M. leprae y (por analogía) que los humanos infectados con el HIV también podrían tener una susceptibilidad incrementada.Nonhuman primates have recently become established as useful animal models for the study of leprosy. Several instances of spontaneous infection have been observed in chimpanzees (Pan troglodytes) (7,12,13) and sooty mangabey monkeys (Cer cocebus atys) (10,15).Recently we have developed a model of Mycobacterium leprae infection using experimentally infected sooty mangabeys (6,8), African green monkeys (Cercopithecus aethiops) (1), and rhesus monkeys (Macaca mulatta) (2,27). These species vary in susceptibility and in the disease pattern which is produced by experimental infection, thus providing an opportunity to study many aspects of the pathogenesis of leprosy.
Simian immunodeficiency virus (SIV) is a lentivirus that is genetically, antigenically, and biologically similar to the human immunodeficiency virus (HIV), the etiologic agent of the acquired immunodeficiency syndrome (AIDS). SIVs occur naturally in African green monkeys, sooty mangabeys, mandrills, and probably other African primates in which they appear to cause persistent but asymptomatic infections. When SIV of mangabey origin is inoculated into macaques, an Asian species, it produces an immunodeficiency syndrome that is very similar in many respects to that produced by HIV infection in humans (4,5,17). SIV infection of macaques is currently the best available animal model for studying lenti-virus-induced immunodeficiency.
There is a high incidence of HIV infection in some geographical areas in which leprosy is also endemic, notably central Africa. AIDS patients have a lowered resistance to M. avium-intracellulare and to M. tuberculosis. Some authors have noted an association between HIV and M. leprae infection (11,14,18), while others have not observed any association (24,26). This may be due to a combination of the slow growth pattern of M. leprae and the early demise of people with AIDS.
We have recently reported an increased susceptibility to M. leprae infection in rhesus monkeys that are also infected with SIV of mangabey origin. The increased susceptibility was associated with a loss of helper-T-cell function and occurred despite antibody response patterns that are usually associated with resistance to M. leprae infection in rhesus monkeys (8,9). We report here the detailed pathological findings relating to M. leprae infection in three of these monkeys which have been examined at necropsy.
MATERIALS AND METHODS
We have inoculated 34 rhesus monkeys with viable M. leprae at various times, using different doses of inoculum and different routes of inoculation. The details of the inoculation procedure have been reported (27). It is likely that some donor tissue and cells remain in the inoculum. Five of these animals (8664, B988, B748,B845, A491) were inoculated with M. leprae from mangabey A022, which was infected with SIV. Rhesus A491 had been previously inoculated with material from mangabey A015, which docs not carry SIV, but did not develop any lesions until after it was inoculated with material from A022 (9). Animals 8664, B988, and A491 became infected with both SIV and M. leprae and died. The remaining two animals did not become infected with cither agent. The clinical, immunological, and serological findings associated with both agents in these animals have been presented elsewhere (9). The general pathologic findings in 8664 and B988 have also been briefly reported, although the lesions due to M. leprae were not detailed (4).
Lepromin skin tests (LST) were done as previously described, and the results read at 4 weeks (Mitsuda reaction) (3).
A complete necropsy examination was performed on the animals that died, with special emphasis on the skin and peripheral nerves. Skin sections were taken from the cars, lips, eyelids, scrotum, and all extremities. The right and left axillary, median, ulnar, sciatic, femoral, and superficial peroneal nerves were sampled. Samples of all major tissues were fixed in 10% neutral buffered Formalin and routinely processed in paraffin for light microscopy. Sections were stained with hematoxylin and eosin (H&E),Masson, and Fite-Faraco acid-fast stains.
RESULTS
Animal 8664. A periorbital B-cell lymphoma was recognized in rhesus 8664 15 months after it was inoculated with mangabcy leproma tissue. At this time, it had cutaneous masses on the scrotum that contained 9.8 x 108 M. leprae per gram of tissue. The monkey was LST negative. Microscopically, the LST test sites showed only slight perivascular cuffing, a few histiocytes, and numerous acid-fast bacilli (AFB), indicating poor clearance. Animal 8664 was euthanatized 21 months after inoculation because of disseminated lymphoma.
At necropsy, there were nodular enlargements of the margins of both cars, and the skin around the nose and upper lip was scaly and nodular. There was a focal ulcerated nodule 1 cm in diameter on the scrotum. Lesions not due to M. leprae consisted of leukemic infiltrates in numerous organs, chronic meningitis, chronic myocarditis, glomerulonephritis, and lymphoid follicular hyperplasia. The lymphoma was of B-cell origin and was associated with rhesus Ep-stein-Barr-like virus (4,20). Microscopic lesions due to M. leprae were documented in the skin (both ears, nose, left wrist, scrotum), peripheral nerve trunks (axillary, ulnar, median, femoral, superficial peroneal), lymph nodes, and nasal mucosa. One histiocyte in the meninges contained AFB. No AFB were observed in the liver, spleen, or bone marrow. Cutaneous lesions consisted of diffuse dermal infiltrates composed of histiocytes intermixed with a few lymphocytes, which sometimes occurred as small islands. There was a distinct subepidermal clear zone. Dermal and subcutaneous nerves were similarly infiltrated. There were abundant solidly stained AFB, singly and in clumps, with large globi (up to 25 microns in diameter) in many histiocytes (Fig. 1). The surface of the scrotal lesions was ulcerated.
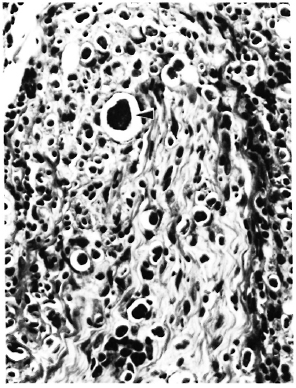
Fig. 1. Section of skin and dermal nerve from rhesus monkey 8664, showing lymphohistiocytic infiltrate and numerous large globi (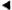 ) (H&E x 250).
) (H&E x 250).
The lesions in the nerves varied from nerve to nerve and between sections of the same nerve, being in general more severe in the distal and more superficial segments. The least severe change consisted of mildto-moderate perivascular infiltration by lymphocytes in the perineurium. No AFB were demonstrable in these lesions. More severe lesions consisted of mild-to-moderate infiltrates of lymphocytes and histiocytes within the nerve. There were numerous AFB in intraneural histiocytes, and occasional AFB in nerve fibers. In addition to follicular hyperplasia and leukemic infiltrates, many peripheral lymph nodes also contained patchy areas of histiocytic infiltration within the sinusoids. These cells often contained globi and numerous AFB.
The nasal submucosa was heavily infiltrated with lymphocytes and histiocytes which contained numerous AFB and globi. Many small mucosal nerves were heavily infiltrated with histiocytes.
Animal B988. Animal B988 was humanely sacrificed 18 months after inoculation because of persistent diarrhea and weight loss that did not respond to treatment. Lesions not due to M. leprae consisted of disseminated cytomegalovirus infection, amyloid deposition in lymph nodes, and lymphoid atrophy.
No changes due to leprosy were apparent grossly, but microscopic changes possibly due to M. leprae were observed in the skin, peripheral nerve trunks, and nasal mucosa. No AFB were seen in the liver, spleen, peripheral lymph nodes, or bone marrow. In many skin sections (scrotum, lip, forearm, thigh) there were minimal-to-mild perivascular lymphocytic infiltrates around some small dermal vessels. No AFB were seen. In other sections (calf, wrist), similar lesions contained a few AFB in small dermal nerves, in mononuclear cells in the perivascular infiltrates, and in the dermis just beneath the epidermis. The skin of the ears, a common site of involvement in monkeys, was normal.
The left and right sciatic and left superficial peroneal nerves contained a few lymphocytes and histiocytes within the perineurium and within the nerve, but no AFB were demonstrated. No inclusion bodies or AFB were seen. There was a dense lympho-histiocytic infiltrate in the nasal submucosa. There were numerous AFB within the infiltrate and within dermal nerves and dermis in the overlying skin.
Animal A491. Rhesus A491 was inoculated twice with mangabey-derived M. leprae, once in December 1980 and again in February 1984. The first donor (mangabey A015) was SIV negative, while the second donor (mangabey A022) was SIV positive. Animal A491 did not develop clinical lesions, except for a few AFB in a nasal smear in April 1982, and was strongly LST positive after the first inoculation, but developed infection with both SIV and M. leprae following the second inoculation. Progressive cutaneous leprosy lesions first appeared in January 1988 (9), and the animal was humanely sacrificed in June 1988.
The monkey had a questionably positive LST reaction in August 1982, although the dose of lepromin used at that time was probably insufficient for an accurate interpretation (3), A LST in February 1983 was strongly positive, producing an ulcerated lesion with focal necrosis, an intense lymphocytic and macrophage infiltrate with epithelioid cell change, and giant cell formation (Fig. 2). A LST in June 1984 was similarly positive. A LST in September 1986 was much smaller, did not ulcerate, and had a sparse unorganized infiltrate with little epithelioid cell change, although there were small foci of necrosis and rare giant cells (Fig. 3).
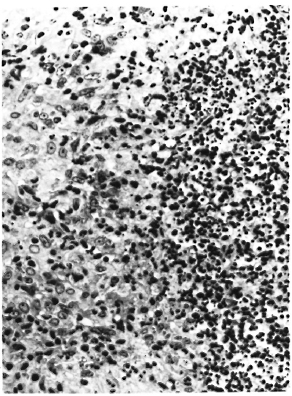
Fig. 2. Positive lepromin skin test (Mitsuda reaction) from rhesus monkey A491 prior to inoculation with SIV-contaminated material. Note delayed-type hypersensitivity granuloma with central necrosis and an intense inflammatory infiltrate (H&E x250).
Fig. 3. Negative lepromin skin test (Mitsuda reaction) from rhesus monkey A491 following inadvertent infection with SIV. Note sparse perivascular histiocytic infiltrate (H&E x250).
At necropsy, the monkey was cachectic, and the perineal skin was reddened and slightly thickened. The skin of the elbows and ankles was thin. The nasal septum was thickened and hemorrhagic. Peripheral nerves were grossly normal.
Lesions not due to M. leprae consisted of retroviral meningoencephalomyclitis and choroid plexitis, bronchopneumonia due to Klebsiella pneumoniae, chronic myocarditis, lymphoid atrophy, cholecystitis and cholangiolitis due to Klebsiella pneumoniae, Cryptosporidium, and Trichomonas, nephrosis, esophagitis, intestinal amyloidosis and cryptosporidiosis, and septicemia due to Klebsiella. Lesions due to M. leprae were seen in the skin, peripheral nerves, peripheral lymph nodes, and nasal mucosa. In addition, Kupffer cells contained rare AFB, although none were observed in the spleen or bone marrow. Every skin section examined contained at least minimal inflammatory changes, which consisted of perivascular and perineural infiltrates of lymphocytes and histiocytes in the dermis. More severely affected sections had mild histiocytic infiltrates around dermal adnexae. There was no epithelioid cell change, and only rare giant cells. Some dermal nerves were heavily infiltrated with histiocytes and many were severely fibrotic with an onionskin appearance to the perineurium and intraneural fibrosis (Fig. 4). Numerous AFB were found in histiocytes, nerves, smooth muscle cells, and endothelial cells of the skin of the nose; whereas in other sections no or only a few or very few AFB were found in the nerves and infiltrate. In some areas AFB were beaded or fragmented, while in others they were solidly stained.
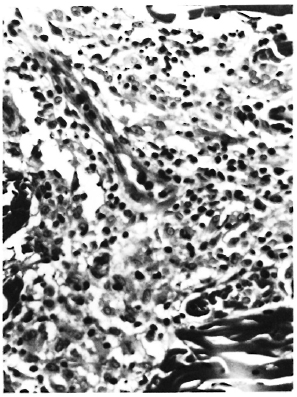
Fig. 4. Dermal nerve from rhesus monkey A491 52 months after SIV infection. Note onion-skin appearance of perineurium and intraneural fibrosis (Masson x 250).
All nerve trunks examined contained mild-to-severe lymphohistiocytic infiltrates within the nerve. Many had a markedly thickened perineurium and some contained increased fibrous connective tissue within the nerve trunk. Many histiocytes contained large globi. There were numerous AFB within histiocytes and nerve fibers in most sections. Most AFB were beaded or fragmented, although a few were solidly stained.
All lymphnodes had severe follicular atrophy and were depleted of lymphocytes. Peripheral nodes often had heavy histiocytic infiltrates in the sinusoids. These cells contained moderate numbers of AFB.
The nasal epithelium was ulcerated and the mucosa diffusely infiltrated with histiocytes, many of which contained globi.
DISCUSSION
As we reported previously, SIV-induced immunosuppression may render rhesus monkeys more susceptible to M. leprae infection probably due to a defect in helper-T-cell activity. SIV infection specifically depletes the subset of T cells that is doubly stained by monoclonal antibodies to OKT4 and 4B4. This cell population contains helper-inducer and memory-T-cell activities (16,23). The lymphoma in monkey 8664 was associated with rhEBV (20). In AIDS patients, EBV-related lymphoma is common and is thought to result from loss of T-cell control of EBV-induced B-cell proliferation (19). It is apparent that the defect that allows EBV and M. leprae to proliferate is present in animals that have lymphoid hyperplasia (8664), as well as lymphoid atrophy and depletion (B988, A491). The defect did not allow M. leprae to disseminate to unusual sites in internal organs, probably due to the preference of At. leprae for cooler temperatures. We and others have observed M. avium-intracellulare (MAI) infections in SIV-infected monkeys and these infections are common in HIV-infected humans, as are M. tuberculosis infections. This further suggests that whatever immune functions are destroyed by primate lentiviruses, they include those that normally prevent the proliferation of mycobacteria.
Both HIV and SIV induce a rash in their respective hosts that is characterized by cutaneous mononuclear inflammatory cell infiltrates and damage to Langerhans' cells, either by direct infection or by an immune-mediated mechanism (22,25). Since Langerhans' cells are important antigen-processing cells in the skin, it is possible that lentivirus infection of these cells might further inhibit an infected individual's ability to control M. leprae.
The three monkeys described here differed in the severity of lesions due to M. leprae. The nasal mucosa and overlying nasal skin were severely affected and contained numerous AFB in all three animals, suggesting that this might be the most susceptible site for M. leprae replication. In B988, only subtle inflammatory changes and occasional AFB were observed at other dermal sites and in nerves. In contrast, monkey A491 had, in addition to nasal lesions, lesions in nerves that appeared more chronic and more severe than those in the dermis. This suggests that M. leprae had been present in nerves for a long time and had only recently begun to spread to the dermis. The extensive neural fibrosis in A491 is compatible with borderline disease, and suggests that A491 did have some immunity to M. leprae during the course of its infection. This apparently downgraded, presumably due to SIV infection, and allowed the lepromatous pattern to develop in the dermis. This is further supported by the declining LST reactivity in this animal. Monkey 8664 had particularly large globi, indicating very little resistance to M. leprae.
The lesions due to SIV in these monkeys were typical of those induced by SIV in other rhesus monkeys. M. leprae infection did not alter the course of SIV infection in any obvious way. This docs not rule out the possibility that M. leprae might act as a co-factor in the induction of immunodeficiency in lentivirus-infected primates, since the whole subject of cofactors is poorly understood.
Although leprosy has not so far increased dramatically in populations in which both M. leprae and HIV are endemic, our studies in SIV- and M. leprae-infectedrhesus monkeys suggest that such an increase should be expected. The increased susceptibility of SIV-infected monkeys to M. leprae may be more readily apparent because of the shorter time course of both diseases in monkeys, and due to the large dose of M. leprae given to experimentally infected monkeys. We believe that an increased incidence of leprosy, particularly of the lepromatous type, will eventually occur in human populations where both HIV and M. leprae are endemic.
Acknowledgments. This work was supported by grants AI-19302 from the National Institutes of Allergy and Infectious Diseases and RR-00164 from the Division of Research Resources, National Institutes of Health. We thank Dorothy Kueblerand Norwood Meiners for technical assistance.
REFERENCES
1. Baskin, G. B., Gormus, B. J., Martin, L. N., Wolf. R. H.. Blanchard, J. L., Malaty, R., Walsh, G. P., Meyers, W. M. and Binford, C. H. Experimental leprosy in African green monkeys (Cercopithecus aethiops):a model for polyneuritic leprosy. Am. J. Trop. Med. Hyg. 37(1987)385-391.
2. Baskin. G. B., Gormus, B. J., Martin, L. N., Wolf, R. H., Murphey-Corb, M., Walsh, G. P., Binford, C. H., Meyers, W. M. and Malaty, R. Experimental leprosy in a rhesus monkey: necropsy findings. Int. J. Lepr. 55(1987)109-115.
3. Baskin, G. B., Gormus, B. J., Martin, L. N., Wolf, R. H., Watson, E. A., Walsh, G. P., Meyers, W. M. and Binford, C. H. The lepromin test in rhesus monkeys. Int. J. Lepr. 54(1986)427-436.
4. Baskin G. B.. Martin. L. N., Rangan, S. R. S., Gormus, B. J., Murphey-Corb, M., Wolf, R. H. and Soike, K. F. Transmissible lymphoma and simian acquired immunodeficiency syndrome in rhesus monkeys. JNCI 77(1986)127-139.
5. Baskin, G. B., Murphey-Corb, M., Watson, E. A. and Martin. L. N. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/Delta. Vet. Pathol. 25(1988)456-467.
6. Baskin. G. B., Wolf, R. H., Gormus, B. J., Martin, L. N., Walsh, G. P., Binford C. H., Meyers, W. M. and Malaty, R. Experimental leprosy in the mangabey (Cercocebus atys): necropsy findings. Int. J. Lepr. 53(1985)269-277.
7. Donham, K. J. and Leininger, J. R. Spontaneous leprosy-like disease in a chimpanzee. J. Infect. Dis. 136(1977)132-136.
8. Gormus, B. J.. Ohashi, D. K., Ohkawa, S., Walsh, G. P., Meyers, W. M., Brennan, P. J. and Trygg, C. Serologic responses to Mycobacterium leprae-specific phenolic glycolipid-1 antigen in sooty mangabey monkeys with experimental leprosy. Int. J. Lepr. 56(1988)537-545.
9. Gormus, B, J., Murphey-Corb, M., Martin, L. N., Zhang, J., Baskin, G. B., Trygg, C. B., Walsh, G. P. and Meyers, W. M. Interactions between simian immunodeficiency virus and Mycobacterium leprae in experimentally inoculated rhesus monkeys. J. Infect. Dis. 160(1989)405-413.
10. Gormus, B. J., Wolf, R. H., Baskin, G. B., OHkawa, S., Gerone, P. J., Walsh, G. P., Meyers, W. M., Binford, C. H. and Greer, W. E. A second sooty mangabey monkey with naturally acquired leprosy: first reported possible monkey-to-monkey transmission. Int. J. Lepr. 56(1988)61-65.
11. Lamfers, E. J. P., Bastiaans, A. H., Mravunac, M. and Rampen, F. H. J. Leprosy in the acquired immunodeficiency syndrome. (Letter) Ann. Intern. Med. 107(1987)111-112.
12. Leininger, J. R., Donham. K. J. and Meyers, W. M. Leprosy in a chimpanzee: postmortem lesions. Int. J. Lepr. 48(1980)414-421.
13. Leininger, J. R., Donham. K. J. and Rubino, M. J. Leprosy in a chimpanzee: morphology of the skin lesions and characterization of the organism. Vet. Pathol. 15(1978)339-346.
14. Meeran, K. Prevalence of HIV infection among patients with leprosy and tuberculosis in rural Zambia. Br. Med. J. 298(1989)364-365.
15. Meyers, W. M.. Walsh, G. P.. Brown. H. L., Binford, C. H., Imes, G. D., Jr., Hadfield, T. L., schlagel, C. J., fukunishi, Y., gerone, P. J., Wolf, R. H„ Gormus. B. J., Martin, L. N., Harboe, M. and Imaeda, T. Leprosy in a mangabey monkey -naturally acquired infection. Int. J. Lepr. 53(1985)1-14.
16. Morimoto, C., Letvin, N. L., Boyd, A. W., Hagan, M.. Brown. H. M., Kornacki, M. M. and Schlossman, S. F. The isolation and characterization of the human helper-inducer T-cell subset. J. Immunol. 134(1985)3762-3769.
17. Murphey-Corb, M.. Martin, L. N., Rangan, S. R. S„ Baskin, G. B., Gormus, B. J., Wolf, R. H., Andes, W. A., West. M. and Montelaro, R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic monkeys. Nature 321(1986)435-437.
18. Pean, C., Pape, J. W., Deschamps, M.-M., Dam-breville, M. and Johnson, W. D., Jr. Natural history of M. leprae and HIV co-infection. (Abstract) In: Proceedings of the V International Conference on AIDS; the Scientific and Social Challenge, Montreal, June 4-9, I9S9. Ottowa: International Development Research Centre, 1989, p. 427.
19. Purtilo, D. T. Opportunistic cancers in patients with immunodeficiency syndromes. Arch. Pathol. Lab. Med. 111(1987)1123-1129.
20. Rangan, S. R. S., Martin, L. N., Bozelka, B. E., Wang, N. and Gormus, B. J. Epstein-Barr virus-related herpesvirus from a rhesus monkey (Macaca mulatto) with malignant lymphoma. Int. J. Cancer 38(1986)425-432.
21. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
22. Ringler, D. J., Hancock, W. W., King, N. W., Letvin, N. L., Daniel, M. D., Desrosiers, R. C. and Murphy, G. F. Immunophenotypic characterization of the cutaneous exanthem of SIV-infected rhesus monkeys. Apposition of degenerative Langerhans cells and cytotoxic lymphocytes during the development of acquired immunodeficiency syndrome. Am. J. Pathol. 126(1987)199-207.
23. Sanders, T. A., Young, H. A. and Shaw, S. Human memory T-lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-γ production. J. Immunol. 140(1988)1401-1407.
24. Sangare, A., Verdier. M., Sorro, B., Ger-shy-Damet, G. M., Leonard, G. and Denis, F. Haute prevalence de L'HTLV-1 et faible prevalence du HIV chez les lepreux de Cote D'lvoire. (Abstract) In: Proceedings of the V International Conference on AIDS; the Scientific and Social Challenge, Montreal, June 4-9, 1989. Ottawa: International Development Research Centre, 1989, p. 175.
25. Tschachler, E., Groh, V., Popovic, M., Mann, D. L., Konrad. K., Safai, B., Eron, L., diMarzo Veronese. F., Wolff, K. and Stingl, G. Epidermal Langerhans cells-a target for HTLV-III/ LAV infection. J. Invest. Dermatol. 88(1987)233-237.
26. Verdier, M., Sangare, A.. Sassou-Guesseau, E., Gaye, A., Al-Qubati, Y. and Denis, F. Etude comparee des prevalences HIV-1, HIV-2, et HTLV-1 chez les lepreaux de 4 pays: Cote D'lvoire, Congo, Senegal, Yemen. (Abstract) In: Proceedings of the V International Conference on AIDS; the Scientific and Social Challenge, Montreal, June 4-9, 1989. Ottawa: International Development Research Centre, 1989, p. 197.
27. Wolf, R. H., Gormus, B. J., Martin, L. N., Baskin, G. B., Walsh, G. P., Meyers, W. M. and Binford, C. H. Experimental leprosy in three species of monkeys. Science 227(1985)529-531.
1. D.V.M., Department of Pathology; Delta Regional Primate Research Center, Tulane University, Three Rivers Road, Covington, Louisiana 70433, U.S.A.
2. Ph.D., Department of Microbiology, Delta Regional Primate Research Center, Tulane University, Three Rivers Road, Covington, Louisiana 70433, U.S.A.
3. Ph.D., Department of Microbiology, Delta Regional Primate Research Center, Tulane University, Three Rivers Road, Covington, Louisiana 70433, U.S.A.
4. Ph.D., Leonard Wood Memorial, Laboratory for Leprosy Research, Everslcy Childs Sanatorium, P.O. Box 727, Cebu City, The Philippines.
5. M.D., Ph.D., Division of Microbiology, American Registry of Pathology. Armed Forces Institute of Pathologv, Washington, D.C. 20306-6000, U.S.A.
Received for publication on 10 October 1989.
Accepted for publication in revised form on 19 December 1989.