- Volume 58 , Number 2
- Page: 389–91
A comparison of the ziehl-neelsen and kinyoun methods in staining smears f rom leprosy patients
To the Editor:
The cold staining method of Kinyoun (3) for acid-fast bacilli is widely used in tuberculosis bacteriology, and is recommended by the U.S.A. Centers for Disease Control (CDC) (2) and the Institut Pasteur, France (1). To our knowledge, this method was not evaluated in leprosy diagnosis. Because the Kinyoun method was applied in our mycobacteriology laboratory, we decided to evaluate it for leprosy diagnosis since this disease is highly endemic in the state of Amazonas, Brazil (estimated incidence and prevalence were 69/100,000 and 11/1000 inhabitants in 1987).
Smears from leprosy patients were prepared in the outpatient leprosy clinic of the Centro de Dermatologia Tropical e Vene-reologia " Alfredo da Matta," Manaus, Brazil. One smear from each site was stained in the outpatient clinic laboratory using the Ziehl-Neelsen method as recommended by Leiker and McDougall (4). The method of Kinyoun was applied as recommended by the CDC and Institut Pasteur (1,2), except that the destaining solution was 1% instead of 3% hydrochloric acid in ethanol. The smears stained by the Ziehl-Neelsen method were read and classified by an experienced microscopist at the outpatient clinic.
Duplicate smears were stained at the Instituto Nacional de Pesquisas da Amazonia (INPA), and were read and classified at INPA. The readings and classifications of the smears were done blindly and independently in the participating laboratories. The results were checked only after complete data were collected.
The two-sample analysis results are shown in Figures 1 and 2. Figure I depicts the frequency polygon of the bacterial index (BI) and morphological index (MI) for all the smears (N = 145). The statistics indicated that there were no significant differences between the two staining procedures. Figure 2 shows the correlation analysis of the paired data on the same smears (N = 145). The correlation coefficients were 0.9 and 0.8, respectively, for the BI and MI, also indicating that there were no differences between the two methods.
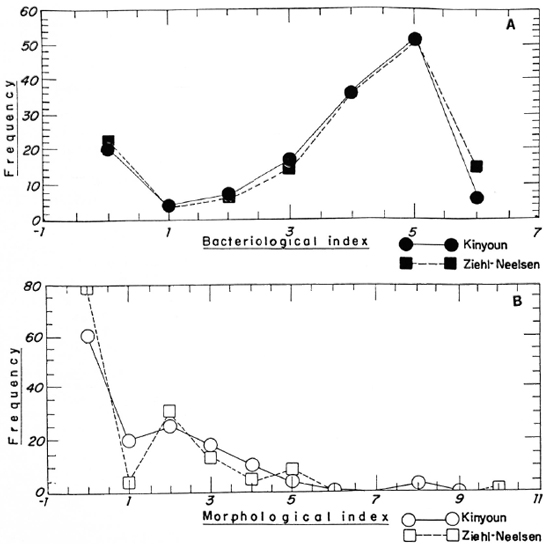
Fig. 1. Frequency polygon showing the frequencies of (A) the bacterial index (Bl) and (B) the morphological index (MI) results usings Ziehl-Neelsen ( and
and  ) and Kinyoun (
) and Kinyoun ( and
and  ) staining procedures. BI significance level 0.488 for α = 0.05; MI significance level 0.403 for α = 0.05. Consequently, do not reject the null hypothesis that there are no differences between the two methods (N = 145).
) staining procedures. BI significance level 0.488 for α = 0.05; MI significance level 0.403 for α = 0.05. Consequently, do not reject the null hypothesis that there are no differences between the two methods (N = 145).
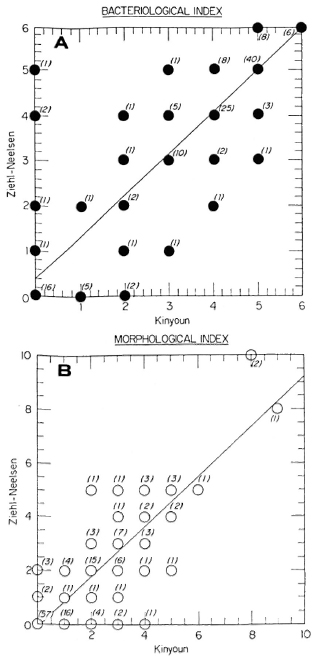
Fig. 2. Regression analysis of paired data for all smears (N = 145). Numbers in parentheses represent how many times each result was equal for the two methods.(A) BI correlation coefficient = 0.88; (B) MI correlation coefficient = 0.87.
From the above data, we conclude that the cold method of Kinyoun for acid-fast microscopy is satisfactory for leprosy bacteriology provided that the concentration of the acid in the destaining solution is reduced to 1%. We have also observed that smears stained with the Kinyoun method keep better in storage, which might be of interest in preparing teaching materials and in quality control programs. One possible disadvantage of the Kinyoun method over the ZiehlNeelsen method is that it uses a higher concentration of fuchsin, which may slightly increase the cost of microscopy.
- F. C. O. Fandinho, Biologist
Investigator
Laboratory of Mycobacteriology
Instituto National de Pesquisas da Amazonia (INPA)
Caixa Postal 478
69.011 Manaus (AM), Brasil
- A. T. Orsi-Souza, M.D.
Chief
Laboratory of Microbiology
Centra de Dermatolagia Tropical Alfredo da Matta
- J. I. Salem, M.D., Ph.D.
Chief
Laboratory of Mycobacteriology
National Research Institute of the Amazonia (INPA)
Acknowledgments. We thank Dr. Hugo David. Institut Pasteur. Paris, for his help in reviewing our data in the preparation of the manuscript, and Mr. Antonio Cauper Filho from the Instituto Nacional de Pesquisas da Amazônia (INPA) for his help with the statistical tests.
REFERENCES
1. David. H. L., Lévy-Frébault, V. and Thorel, M. F. Méthodes de Laboratoire pour Micobactério-logie Clinique. Comission des Laboratoires de Référence et d'Expertise de l'Institut Pasteur, eds. Paris: Institut Pasteur, 1989.
2. Kent, P. T. and Kubica, G. P. Public Health Mycobacteriology; a Guide for the Level III Laboratory. Atlanta; Centers for Disease Control, 1985.
3. Kinyoun. J. J. A note on Uhlenhuth's method for sputum examination for tubercle bacilli. Am. J. Pub. Health 5(1915)867-870.
4. Leiker, D. L. and McDougall, A. C. Technical Guide for Smear Examination for Leprosy by Direct Microscopy. Amsterdam: Leprosy Documentation Service (INFOLEP). 1983.