- Volume 57 , Number 4
- Page: 735–43
PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy
ABSTRACT
Multibacillary (MB) and paucibacillary (PB) leprosy patients were tested for circulating phenolic glycolipid-I (PGL-I) antigen and antibodies before treatment. In the 27 MB patients tested, 27 (100%) were antigen positive with levels ranging f rom 50 to 5000 ng/ml, and 26 (96%) were antibody positive with titers ranging f rom 1000 to 64,000. Although the antigen and antibody levels were much higher in MB than in PB patients, we could not demonstrate a correlation between the number of acid-fast bacilli/mg of skin biopsy and these two parameters in 14 MB patients. After starting daily multidrug therapy, 10 MB patients were monitored monthly. As much as 88.75% ± 10.8% of the PGL-I antigen was cleared f rom the bloodstream after 1 month while the anti-PGL-I antibody remained stable. This rapid decrease in the PGL-I antigen level strongly suggcsts the usefulness of this test for monitoring MB patients under chemotherapy.RÉSUMÉ
Chez des malades atteints de lèpre multibacillaire (MB) et paucibacillaire (PB), on a mesuré, avant traitement, les taux de l'antigène circulant phéno-glyco-lipidique-I (PGL-I), et les taux d'anticorps. Chez les 27 sujets multibacillaires qui ont été étudiés, tous étaient positifs pour l'antigène, avec des taux variant de 50 à 5000 ng/ml, et 26 d'entre eux (soit 96%), avaient des anticorps dont les titres s'étalaient entre 1000 et-64.000. Quoique les taux de l'antigène et les taux d'anticorps atteignaient des valeurs beaucoup plus élevées chez les multibacillaires que chez les paucibacillaires, il n'a pas été possible chez 14 malades multibacillaires de mettre en évidence une corrélation entre le nombre de bacilles acido-résistants par mg de biopsie cutanée et ces deux paramètres. Dix patients multibacillaires ont été suivis mensuellement à partir du moment où ils ont été placés sous polychimiothérapie quotidienne. Après 1 mois 88,75% ± 10% de l'antigène PGL-I avait disparu de la circulation, alors que le taux de l'anticorps anti-PGL-I restait stable. Cette diminution rapide du taux d'antigène PGL-I suggère fortement que cette épreuve pourrait être très utile pour suivre les malades multibacillaires sous chimiothérapie.RESUMEN
Se buscó la presencia del antígeno glicolípido fenólico-I (PGL-I) y de anticuerpos anti-PGL-I en la circulación de pacientes con lepra multibacilar (MB) y paucibacilar (PB), antes y después de tratamiento. De los 27 pacientes MB estudiados, 27 (100%) fueron positivos para antígeno con niveles entre 50 y 5000 ng/ mi, y 26 (96%) fueron positivos para anticuerpo con títulos de 1000 a 64,000. Aunque los niveles de antígeno fueron mucho más altos en los pacientes MB que en los PB, no pudimos demostrar una correlación entre el número de bacilos ácido resistentes por mg de biopsia de piel y estos dos parámetros en 14 pacientes MB. Después de empezar una terapia diaria con múltiples drogas, 10 pacientes MB fueron evaluados mensual-mente. Se encontró que después de 1 mes de tratamiento, el 88.75% ± 10.8% del antígeno PGL-I fue eliminado de la circulación mientras que el anticuerpo anti-PGL-I permaneció estable. Esta rápida disminución en el nivel de antígeno indica la utilidad de esta prueba en el seguimiento de pacientes MB bajo tratamiento.Antigen detection in leprosy has been developed recently, and the test for phenolic glycolipid-I (PGL-I) antigen detection, in either the serum or urine of lepromatous patients, has been described (1,6,11,18). PGL-I antigen is synthesized and excreted by Mycobacterium leprae (10), and has been demonstrated to be cleared sooner than the corresponding antibody in the sera of patients undergoing multidrug therapy (MDT). This suggests that glycolipid quantitation probably correlates more closely with the current infection than do antibodies (6), and thus suggests the possible usefulness of PGL-I detection in monitoring the effectiveness of a therapy.
In the present paper, we report the results obtained for PGL-I antigen (Ag) and anti-PGL-I antibodies (Ab) in multibacillary (MB) and paucibacillary (PB) leprosy patients, correlations with the bacillary load, and variations during MDT.
MATERIALS AND METHODS
Negative control sera
Negative control sera were obtained from healthy individuals living in a nonendemic area (France) and from healthy Polynesians without known contact with leprosy.
Patients and treatment
The sera of 41 leprosy patients-14 lepromatous (LL), 2 borderline lepromatous (BL), 12 indeterminate (I), 6 borderline tuberculoid (BT), 7 tuberculoid (TT)-attending the outpatient clinic of the Institut Louis Malarde, Papeete, Tahiti, were collected before treatment. The diagnosis of leprosy was based on the results of the clinical examination, supplemented by biological tests: intradermal lepromin reaction, acid-fast bacilli (AFB) in the nasal mucosa and the skin (lesion and two earlobes), and biopsy for histopathological examination (P. Ravisse, Institut Pasteur, Paris, France). Moreover, since 1982, a biopsy for mouse inoculation and drug sensitivity testing was performed. Twenty-one patients from New Caledonia, diagnosed according to the same criteria (9 LL, 2 BL, 6 I, 1 BT, 3 TT) were also tested before treatment. All of these patients were assigned into a paucibacillary (PB) or a multibacillary (MB) group according to the Ridley-Jopling classification (14). The bacillary load (number of AFB per mg of tissue) before treatment, available for 14 MB patients from Tahiti, was determined at the time of the inoculation into the mouse foot pad for drug sensitivity testing (Prof. J. Grosset, Hôpital Pitié-Salpêtrière, Paris, France).
Until 1982, dapsone (DDS) monotherapy was the basis of the treatment prescribed for 10 years, on the average, for PB patients (I, TT and BT) and lifelong for MB patients (BB, BL and LL). Since 1982, a multidrug therapy has been implemented in French Polynesia consisting of: for PB patients, daily administration for 6 months of 100 mg of DDS and 10 mg/kg of rifampin (RMP); for MB patients, daily administration for 24 months of DDS and RMP in the same doses as for PB with a daily supplement of 100 mg of clofazimine during the first 12 months and 5 mg/kg of prothionamide during the first 2 months.
Anti-PGL-I IgM by ELISA
IgM anti-PGL-I antibody was quantitat-cd by ELISA according to a method derived from Cho, et al. (5) and previously described (4).
Briefly, the antigen used was the semisynthetic natural trisaccharidc 3-p-hydroxy-phenyl-propionate (NTP) coupled to bovine serum albumin (Lot FUJI-XI-66-860717; Institute for Natural Science, Nara University, Japan) (7). NTP antigen was coated to flat-bottom EIA microplates (CEB-CML, Nemours, France). The nonspecific binding of the sera was measured by control wells not coated with antigen. The sera were diluted to 1/250 and tested in duplicate antigen and control wells. After washing, goat anti-human IgM peroxidase conjugate (BIOSYS, Compiegne, France) was added and the plates were incubated. The reaction was revealed when the chromogenic substrate orthophenyl-ethylene-diamine (OPD; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) was added with H2O2 solution at 0.013% final concentration. Automatic optical density (OD) readings and calculations were performed by a micro-ELISA reader (Titertek; Flow Laboratories, Helsinki, Finland). The results are expressed as the difference in OD between the antigen-coated and control wells (ΔOD).
A serum was considered "positive" when the ΔOD exceeded by two standard deviations (S.D.) the mean ΔOD obtained from normal Polynesian sera at the same dilution. The cut-off point was 0.194 rounded to 0.200 for convenience. The positive sera were titrated. The titer is the reciprocal of the last dilution giving a positive result.
Semiquantitative PGL-I detection by DOT-ELISA
We used the methodology of Cho, et al. (6) with minor modifications. To prevent contamination, all glassware used in this test was disposable. The different solvent phases were evaporated in a heat-controlled evaporator equipped for 32 tubes and connected to nitrogen gas (Liebisch-Bioblock, Paris, France).
PGL-I extraction and purification procedures. The PGL-I was extracted from 500 μl of lyophilized serum by 5 ml of chloro-form/methanol 2/1 overnight at 45ºC. The chloroform/methanol phase was evaporated under nitrogen at 45ºC, and the extract so obtained was purified on a small florisil column (0.5 cm diameter, 5 cm length). The first chloroform phase was discarded, and the PGL-I was desorbed by 7 ml of chloroform/mcthanol 95/5, dried under nitrogen, and stored ready to be quantitated by DOT-ELISA.
To check the efficiency of the extraction procedure, each series of extractions included a control made of normal serum to which was added a known amount of purified PGL-I (Colorado State University, Fort Collins, Colorado, U.S.A.).
DOT-ELISA on nitrocellulose membrane. The PGL-I extract was dissolved in 100 μl of hexane (i.e., it was 5 times concentrated as compared to normal serum) and then serially twofold diluted. Five μl of each dilution was absorbed on nitrocellulose (NC) membrane previously soaked in distilled water and dried. The excess of the reactive sites was blocked with phosphate buttered saline containing 5% bovine serum albumin (PBS-BSA) for 1 hr at 37ºC. The NC membrane was incubated with anti-PGL-I hyperimmune rabbit serum (Colorado State University) for 1 hr at 37ºC, rinsed 4 times with PBS, and incubated with peroxidase conjugate anti-rabbit IgG for 1 hr at 37ºC.
After 5 washings, 4-choloronaphthol 0.05% containing H2O2 0.009% was added as substrate. A positive result appears as a blue-purple dot after 20 min at 37ºC. The last concentration or dilution giving a positive result was noted.
As a standard, serially diluted purified PGL-I was applied in a known amount on the NC membrane; this method allows the detection of 30 ng/ml. The amount of PGL-I contained in each serum was calculated according to this standard and the last concentration or dilution of the extract giving a positive result. All of the results were expressed in nanograms of PGL-I per ml of serum. It was therefore possible to detect as little as 6 ng of PGL-I/ml due to the 5 times concentration of the extract, but a serum was considered "positive" for PGL-I Ag when the concentration was > 12 ng/ml.
RESULTS
Negative control subjects
For anti-PGL-I IgM, none of the 40 healthy French individuals and 4 of the 93 healthy Polynesians were positive; mean Ab levels ± 2 S.D. were ΔOD = 0.010 ± 0.036 and ΔOD = 0.054 ± 0.140, respectively.
For PGL-I Ag, all of the 20 French controls and the 50 healthy Polynesian controls had undetectable levels of PGL-I Ag in their sera (< 6 ng/ml).
Patients before treatment
PB patients. Before treatment, 35 sera of PB patients (18 I, 7 BT, 10 TT) were tested (Table 1): 12 (34%) were Ab positive (titers ranged from 250 to 1000, and ΔOD at 1/250 dilution of the sera ranged from 0.200 to 0.600) and 23 (66%) were Ab negative; 7 (20%) were Ag positive and 28 (80%) were Ag negative. Out of the 12 Ab-positive patients, 4 (33%) were Ag positive. Out of the 23 Ab-negative patients, 3 (13%) were Ag positive. When detectable, PGL-I levels in the PB patients ranged from 12 ng/ml (limit of positivity of the test) to 50 ng/ml. The results of the PGL-I Ag levels are shown in The Figure.
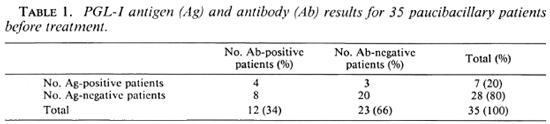
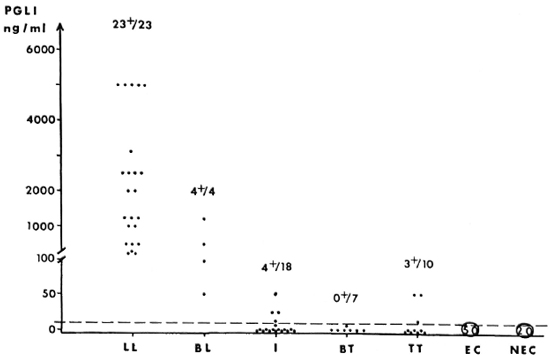
The Figure. PGL-I antigen distribution in sera of leprosy patients across the disease spectrum. EC = endemic negative controls; NEC = nonendemic negative controls.
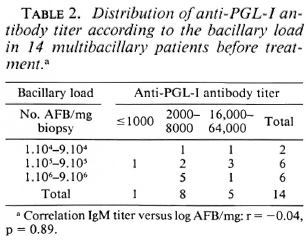
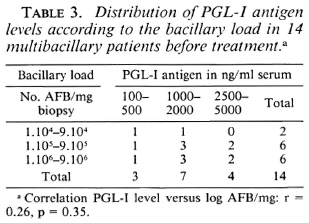
MB patients. Twenty-seven sera of MB patients (4 BL, 23 LL) were tested: 26 (96%) were Ab positive (titers ranged from 1000 to 64,000, and ΔOD at 1/250 dilution of the sera was > 0.600) and only one was Ab negative; all of the 27 (100%) MB patients were Ag positive (levels ranged from 50 to 5000 ng/ml). The MB patients displayed significantly higher PGL-I levels than did the PB patients (p < 0.001). The Ag levels of the 27 MB patients are plotted in The Figure. The bacillary load (number of AFB per mg of biopsy) before treatment was available for only 14 of these MB patients tested. The distribution of Ab titer for these patients according to the bacillary load is shown in Table 2. The coefficient of correlation between Ab titer and the log of the number of AFB per mg was r = -0.04, p = 0.89. The distribution of the Ag levels according to the bacillary load is shown in Table 3. We could not find significant correlations between the Ag concentration and the log of the number of AFB per mg of tissue (r = 0.26, p = 0.35) or between the Ag concentration and the Ab titer (r = -0.08, p = 0.78).
Patients under daily MDT
PB patients. Three to 12 months after starting MDT, 12 sera could be collected from the 35 PB patients tested before treatment. One of the 12 sera was from an initially Ab-positive patient and was still Ab positive; the other 11 sera were from initially Ab-ncgative patients and remained Ab negative. Of these 12 sera, 4 were Ag positive when previously tested, and only the one Ab-positive patient remained Ag positive. In this patient, the Ag increased from 50 ng/ml to 120 ng/ml, and a downgrading reaction (from borderline tuberculoid to borderline lepromatous type) was diagnosed. The other eight patients, who were previously Ag negative, remained negative.
MB patients. Among the 35 MB patients on MDT, 27 sera were collected before treatment, 29 between 1 to 12 months, 13 between 13 to 34 months, and 11 between 25 to 73 months after beginning MDT. The mean Ab activity at 1/250 dilution of the sera and the mean Ag level were calculated for each group of sera. The reduction of Ab and Ag after MDT was calculated as compared to the corresponding value obtained before treatment (Table 4). Antigen decreased 92% in the group of sera collected between 1 to 12 months after beginning MDT. The antibody decrease was much lower than the antigen decrease.
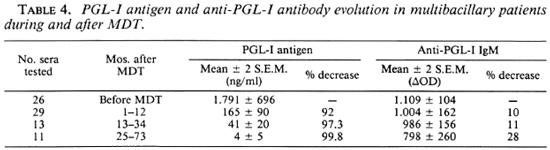
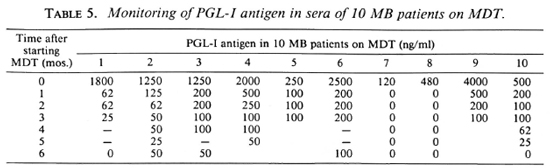
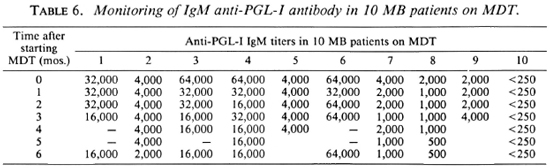
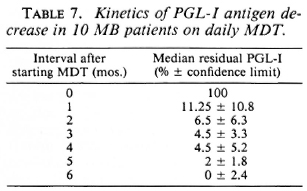
In order to assess more accurately the kinetics of anti-PGL-I antibody and PGL-I antigen variations in the sera, 10 MB patients (8 LL, 2 BL) have been monitored monthly during MDT. The individual results are reported in Table 5 for Ag and in Table 6 for Ab. For each patient, the percentage of residual PGL-I Ag was calculated compared to its initial level before treatment, and the median of these percentages was determined from month 1 to month 6 after inception of MDT (Table 7). The median of the percentage PGL-I Ag decrease was 88.75 ± 10.8% at 1 month, 95.5 ± 3.3% at 3 months, and 100 ± 2.4% at 6 months. Conversely, Ab titers varied inconsistantly, depending on each patient: at month 6 the decrease can be 0, 1, or a maximum of 2 dilutions of the sera. The Ab geometric mean titer for these 10 patients was 6987 before treatment, 4591 at month 3, and 4000 at month 6 after inception of MDT.
Patients under long-term DDS monotherapy
Twenty MB patients on DDS monotherapy for 20 to 40 years who had no sign of relapse were tested for Ag and Ab: 3 (15%) of them were still Ab positive. No differences could be found, according to the duration of treatment, between the 3 seropositive and the 17 seronegative subjects, all of whom were Ag negative.
Apart from these 20 subjects, two MB patients on DDS monotherapy suffered a relapse, clinically, bacteriologically and histopathologically confirmed. At the time the relapses were diagnosed, the Ab levels in these two cases were ΔOD = 1.056 and δOD = 0.116 and the Ag levels were 1000 ng/ml and 500 ng/ml, respectively.
DISCUSSION
An antibody test performed with NTP antigen was positive for 34% of the PB patients and 96% of the MB patients before treatment. The antibody level was much lower in PB patients than in MB patients suggesting, as reported by several authors, that antibody production correlates with the bacterial load of the patient (2,3,12). However, in the MB form of the disease we observed a wide range of antibody responses among the 26 Ab-positive individuals; titers varied from 1000 to 64,000. From the results we obtained for 14 of these MB patients, we could not demonstrate a significant correlation between antibody production and the number of AFB in the tissue (r = -0.04, p = 0.89). Some genetic factors relative to each individual may influence the antibody production and may likely explain this variation, as demonstrated in the mouse model infected experimentally with M. lepraemurium (9). A larger number of patients will have to be studied to confirm these results.
Using the technique we described for PGL-I antigen detection, all of the negative control sera (20 French and 50 Polynesian) were negative, demonstrating the great specific ity of the test. When patients were tested across the disease spectrum, the MB patients displayed the highest levels of circulating PGL-I and the PB patients showed the lowest levels. Of the 35 PB patients, 7 (20%) had PGL-I antigen detectable at a low level (12 to 50 ng/ml) in their sera before treatment. Among this group of patients, the same proportion of positive subjects was found in the patients with indeterminate leprosy (4 out of 18) as in the patients with BT/TT leprosy (3 out of 17). Antigen detection, under certain conditions, may thus help to confirm the diagnosis of indeterminate leprosy when the clinical, bacteriological and histopathological critera for diagnosis are not convincing. These results also suggest that such a test would be sensitive enough to detect the presence of a very small number of M. leprae. This may be explained by the lipidic capsule of phenolic glycolipid formed around the bacilli (10).
Among the MB patients, the BL patients seemed to display lower levels of PGL-I than did the LL patients. In the LL patients, the antigen levels before treatment varied up to 40-fold (120 to 5000 ng/ml) and did not correlate with antibody levels (r = -0.08, p = 0.78). Since all of the antigen trapped in immune complexes in the serum are extracted using the technique described, the absence of correlation (which however needs to be confirmed with more patients) cannot be assigned to this phenomenon. The bacillary load before MDT was available for only 14 of the 27 MB patients tested, and we could not find a significant correlation between antigen concentrations in the sera and the number of AFB determined from skin lesions (r = 0.26, p = 0.35). (We need to complete this study with a larger number of patients.) However, several reasons may explain this observation: a) the variability of the AFB distribution in the tissues of a patient; b) the PGL-I level is likely to be correlated with the number of viable bacilli secreting antigen and not with the total number of AFB; and c) PGL-I measured in the serum is a better expression of all the viable bacilli present in an individual, and probably is more reliable than the enumeration of total AFB in one lesion. Certainly, PGL-I concentration in the tissues correlates closer with the bacillary load of the same biopsy (16) than does the PGL-I level in the serum.
In 10 MB patients undergoing daily MDT, the amount of PGL-I in the serum declined by 88.75% as early as 1 month after the inception of treatment. Concerning antibody evolution, a 6-month time span was too short to obtain a significant antibody decrease. Thus, the PGL-I antigen test seems able to provide an early reflection of the effectiveness of chemotherapy. These results suggest that for certain purposes this type of assay may have an application and be of practical importance in monitoring patients' responses to treatment. Laboratory investigation concerned with the bacteriological aspect of leprosy patients, whether for diagnosis or monitoring responses to treatment, depends on a very limited choice of techniques: a) the bacterial index (BI), and b) the morphological index (MI) in specimens from skin lesions or nasal mucosa. These routine techniques have been difficult to use under field conditions and to standardize from center to center. Because of the operational difficulties in using the MI, this marker is no longer recommended for routine activities (17). At the present stage of the technology, PGL-I antigen detection is not suitable as a routine technique for the surveillance of patients in endemic countries. For research purposes, however, PGL-I detection would represent some advantages: a) antigen level declines much faster than the BI, which decreases only 0.6 log10 per year during regular treatment (8), and b) the MI is hardly accurate but technically demanding, requiring precise staining conditions and a skilled staff (13).
In two long-term-treated MB patients suffering a relapse, PGL-I antigen could be detected in both. The possible supportive role of the PGL-I antigen test in predicting relapse of multibacillary leprosy should be studied further.
In MB patients treated with a single dose of 600 or 1500 mg of RMP, a decrease of more than 99% of the viable bacilli was observed within a few days (15). Our results showed a PGL-I decrease of only 89% after 1 month of daily MDT. This discrepancy may be due to the secretory nature of PGL-I and its persistence as a capsule around the bacilli. The delay in the clearance of circulating antigen may be explained by the large amount of PGL-I accumulated in the tissues that is released slowly into the serum (10) and then contributes to maintain a residual antigenemia. The concentration of PGL-I in the bloodstream may not then correspond to the number of viable bacilli at a given time.
In the matter of research programs to be undertaken, the development of sensitive tools to evaluate the efficacy of chemotherapy and of new drugs is an important point. At the present time, this evaluation is based on a) clinical observation, b) determination of the BI and MI and, more importantly, the enumeration of the viable AFB using inoculation into the mouse foot pad, and c) the occurrence of relapses. Because of the absence of techniques for culturing M. leprae in vitro, the in vivo mouse foot-pad technique is the only available tool for testing drug sensitivity and drug resistance. This test is technically difficult to perform, invasive for the patient because of the serial biopsies needed, and the results are available only about 1 year later. Therefore, a relatively simple and rapid test such as PGL-I detection may constitute an interesting tool.
A comparative study between the kinetics of circulating PGL-I clearance and the decrease in the number of viable M. leprae using inoculation into the mouse foot pad during therapy would greatly clarify the respective advantages of these two techniques to assess the effectiveness of a drug regimen.
Acknowledgments. This research was supported by the Ministère de l'Enseignement Supérieur et de la Technologie (décision no. 86.S.0682), Institut Pasteur de Paris, and Fondation Raoul Follereau.
We thank Prof. P. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A., for providing the PGL-I antigen and antibody through funds from NIAID (contract no. l-AI-52582) and Dr. T. Fujiwara, Nara University, Nara, Japan, for providing thc NTP antigen.
We kindly acknowledge P. Luquiaud and C. Plichart for their technical assistance and J. P. Boutin for his advice on statistics.
REFERENCES
1. Aguado Sanchez, G., Malik, A., Tougne, C, Lambert, P. H. and Engers, H. D. Simplification and standardization of serodiagnosis tests for leprosy based on phenolic glycolipid-I (PGI) antigen. Lepr. Rev. 57Suppl. 2(1986)83-93.
2. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid I and to whole M. leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
3. Buchanan, T. M., Young, D. B., Miller, R. A. and Khanolkar, S. R. Scrodiagnosis of infection with M. leprae. Int. J. Lepr. 51(1984)524-530.
4. Chanteau, S., Cartel, J.-L., Roux, J., Plichart, R. and Bach, M.-A. Comparative study of a di-saccharide and two disaccharide synthetic antigens for detection of anti-PGL-I antibodies in leprosy patients and their household contacts. J. Infect. Dis. 157(1988)770-776.
5. Cho, S.-N., Fujiwara, T., Hunter, S. W., Rea, T. H., Gelber, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-β-d-glucopyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-321.
6. S.-N., Hunter, S. W., Gelber, R. H., Rea, T. H. and Brennan, P. J. Quantitation of the phenolic glycolipid of M. leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153(1986)560-569.
7. Fujiwara, T. and Izumi, S. Synthesis of the neo-glycoconjugates of phenolic glycolipid related tri-saccharides for the serodiagnosis of leprosy. Agric. Biol. Chem. 51(1987)2539-2545.
8. Grosset, J. H. Recent developments in the field of multidrug therapy and future research in chemotherapy of leprosy. Lepr. Rev. 57Suppl. 3(1986)223-234.
9. Hoffenbach, A., Diioro, L. and Bach, M.-A. Influence of route of inoculation on anti-M. leprae antibody isotypes in murine leprosy. Int. J. Lepr. 55(1987)305-315.
10. Hunter, S. W., Fujiwara, T. and Brennan, P. J. Structure and antigenicity of the major specific glycolipid antigen of M. leprae. J. Biol. Chem. 257(1982)15072-15078.
11. Kaldany, R. R., Maasho, K., Ohman, R., Reitz-Vick, D., Britton, S. and Lefford, M. J. Methods for the detection of a specific Mycobacterium leprae antigen in the urine of leprosy patients. Scand. J. Immunol. 25(1987)37-43.
12. Meeker, H. C, Levis, W. R., Sersen, E., Schuller-Levis, G., Brennan, P. J. and Buchanan, T. M. ELISA detection of IgM antibodies against phenolic glycolipid-I in the management of leprosy: a comparison between laboratories. Int. J. Lepr. 54(1986)530-539.
13. Ridley, D. S. The morphological index. Lepr. Rev. 42(1971)75-77.
14. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
15. Shepard, C. C, Levy, L. and Fasal, P. Further experience with the rapid bactericidal effect of rifampin on M. leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1124.
16. Venkatesan, K., Singh, H., Bharadwaj, V. P. and Ramu, G. Isolation, purification and quantification of phenolic glycolipid-1 from human leprosy skin tissues. Trans. R. Soc. Trop. Med. Hyg. 82(1988)321-323.
17. WHO Expert Committee on Leprosy. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
18. Young, D. B., Harnisch, J. P., Knight, J. and Buchanan, T. M. Detection of phenolic glycolipid-I in sera from patients with lepromatous leprosy. J. Infect. Dis. 152(1985)1078-1081.
1. Ph.D.; Institut Territorial de Recherches Medicales Louis Malarde, B.P. 30, Papeete, Tahiti, French Polynesia.
2. M.D.; Institut Territorial de Recherches Medicales Louis Malarde, B.P. 30, Papeete, Tahiti, French Polynesia.
3. M.D.; Institut Territorial de Recherches Medicales Louis Malarde, B.P. 30, Papeete, Tahiti, French Polynesia.
4. Computer Scientist; Institut Territorial de Recherches Medicales Louis Malarde, B.P. 30, Papeete, Tahiti, French Polynesia.
5. M.D., Institut Territorial de Recherches Medicales Louis Malarde, B.P. 30, Papeete, Tahiti, French Polynesia.
6. Chemist, Institut Pasteur, Noumea, New Caledonia.
Received for publication on 29 December 1988.
Accepted for publication in revised form on 16 May 1989.