- Volume 57 , Number 4
- Page: 744–51
Serum antibodies to defined carbohydrate antigens during the course of treated leprosy
ABSTRACT
Sequential monitoring of 724 sera for antibodies to a neoantigen based on phenolic glycolipid-I (PGL-I) and native lipoarabi-nomannan (LAM) in 90 leprosy patients undergoing therapy in San Francisco was conducted. Untreated lepromatous patients frequently (91%) had significant antibodies to both moieties. Antibodies were less frequently found in tuberculoid patients (74% to neoantigen and 37% to LAM). In the first 3 years of treatment, average serum antibodies to both moieties fell significantly. Antibodies to LAM fell during each of the first 4 years of therapy, but decreasing antibody levels to the PGL-I neoantigen did not appear to fall consistently after the third year of treatment. A wide variation in the rate of fall of serum antibodies was noted. Sequential changes in the amounts of serum antibodies to the neoantigen and LAM in general paralleled one another but were at times discrepant. Both in San Francisco and Malaysia, skin-smear negative, long-term treated, lepromatous leprosy patients frequently harbored significant antibodies to both PGL-I and LAM.RÉSUMÉ
A San Francisco, che/ 90 malades traités pour la lèpre, on a mené une étude consistant à doser des anticorps à un néoantigène basé sur l'antigène phénogly-colipidique I (PGL-I) et au lipoarabinomannan (LAM) dans 724 échantillons de sérum obtenus de manière séquentielle. Le plus souvent (91% deseas), les malades lépromateux non traités présentent des taux significatifs d'anticorps à ces deux antigènes. Des anticorps étaient moins fréquemment observés chez des malades tuberculoïdes (74% pour le néoantigène et 37% pour le LAM). Au cours des trois premières années de traitement, le taux moyen d'anticorps sérique à ces deux antigènes a décru de manière significative. Les taux d'anticorps au LAM ont diminué de manière régulière au cours des quatre premières années de la thérapeutique; par contre les taux d'anticorps au néoantigène PGL-I n'ont pas semblé décroître de manière régulière après la troisième année de traitement. On a observé une large variation dans les taux de chute des anticorps sériques. La succession des modifications dans les taux d'anticorps sériques au néoantigène et au LAM étaient généralement similaires, encore qu'à l'occasion elles aient montré des différences. Tant à San Francisco qu'en Malaisie, les patients lépromateux, négatifs aux frottis cutanés et traités pendant une longue période de temps, témoignent souvent de taux significatifs d'anticorps à la fois contre le PGL-I et contre le LAM.RESUMEN
Se hizo un estudio (en San Francisco) de seguimiento en 724 muestras de suero de 90 pacientes con lepra en el que se investigó la presencia de anticuerpos contra un neoantígeno basado en elglicolípido fenólico-1 (PGL-I) y contra la lipoarabinomanana (LAM) nativa. Los pacientes lepromatosos no tratados, frecuentemente (91%) tuvieron anticuerpos a niveles significantes contra ambos determinantes. Los anticuerpos se encontraron menos frecuentemente en los pacientes tuberculides (74% contra el neoantigeno y 37% contra LAM). El promedio de anticuerpos séricos contra ambos determinantes cayó significativamente después de los primeros 3 años de tratamiento. Los anticuerpos contra LAM cayeron cada año durante los primeros 4 años de tratamiento, pero los niveles de anticuerpo contra el I'GL-1 no parecieron disminuir consistentemente después del tercer año de tratamiento. Se observó una amplia variación en la velocidad de caída de los anticuerpos séricos. Los cambios secuenciales en la cantidad de anticuerpos séricos contra el neoantígeno y contra LAM, en general fueron paralelos aunque algunas veces fueron discrepantes. Tanto en San Francisco como en Malasia, los pacientes con tiempos prolongados de tratamiento y bacteriológicamente negativos tuvieron niveles significantes de anticuerpos contra cl PGL-I y contra LAM.In 1983 we (5) described a serologic test for leprosy infection based on the presence in Mycobacterium leprae of a unique constituent, phenolic glycolipid (PGL-I) (8,9). The presence of serum antibody to PGL-I has proved to be highly specific for infection with M. leprae; patients with tuberculosis and other mycobacterial infections only rarely (4%) harbor antibody (5). At the lep-romatous end of the leprosy spectrum antibody to PGL-I appears highly sensitive, with 91 %-96% of untreated patients harboring IgM antibody (4,5,7,20). By contrast, in tuberculoid leprosy fewer (27%-62%) patients have antibodies to PGL-I (4,5,7,20).
In our earliest studies we found that lep-romatous leprosy patients treated for over 2 years harbored IgM antibody to PGL-I less commonly than those treated for less than 2 years, and their mean antibody levels were lower (5). Also, in a small group of leprosy patients undergoing therapy, whose antibody levels to the PGL-I were studied on several occasions, antibody levels, fell consistently over time (5). Others (1,6,11-13) have now noted a correlation between the number of organisms present in the skin, the level of antiglycolipid antibodies, and the level of anti-lipoarabinomannan antibodies. Unlike PGL-I, lipoarabinomannan (LAM) is common throughout the mycobacterial genus (10). Furthermore, others have also found that the levels of antibodies to these two major carbohydrate antigens decreased after initiation of effective chemotherapy (1,6,11-13). Our present studies were conducted to examine further the changes in antibody during the course of treated leprosy and, specific ally, to determine if these changes could prove of assistance to clinicians managing these patients.
MATERIALS AND METHODS
Patient populations studied. Sera, 724 in number, were obtained sequentially from 90 patients attending the San Francisco Regional Hansen's Disease Clinic between 1982 and 1986. The number of sera obtained from each patient averaged 8, ranging from 2-21. In the main, patients were selected for the study either because therapy was initiated in 1982 or because the patients underwent a significant reactional state sometime during these years. All patients were classified clinically and histopatholog-ically according to the method of Ridley and Jopling as borderline tuberculoid (BT), 24; borderline lepromatous (BL), 12; or lepro-matous (LL), 54. Patients were treated entirely on an outpatient basis. Generally the BT patients received daily monotherapy, 50-100 mg of dapsone, and the BL and LL patients daily 50-100 mg of dapsone and daily 600 mg of rifampin.
Additionally, a single serum from each of 45 patients attending the San Francisco Regional Hansen's Disease Clinic known to be skin-smear negative in six disparate sites, and who were originally classified as having BL or LL leprosy, was analyzed for antibody to the neoantigen and LAM. These patients had been receiving antimicrobial therapy for leprosy for an average of 10.5 years (6 to 35 years) previously. These patients were treated either with monotherapy, generally daily dapsone, or combination chemotherapy, generally daily dapsone and rifampin.
Lastly, a set of two paired sera were obtained from a group of Malaysian patients, both with active lepromatous leprosy (bacterial index 3.0-5.0) in 1972-1976 and at a time of smear negativity in 1986. These paired sera were assessed for the presence of antibody to the neoantigen and LAM. These patients were institutionalized during this time, and their therapy was supervised. Patients received continuous specific anti-leprosy therapy consistently. In the minority of cases treatment consisted of only monotherapy dapsone or clofazimine. However, most of these patients were treated with combination chemotherapy consisting of two or more of the following agents: dapsone, clofazimine, rifampin, and thiam-butosine.
Antigens and conditions for ELISA. The synthesis and serological activity of the PGL-I neoantigen has been described (3). The full chemical title is O-(3,6-di-O-methyl-β-D- glucopyranosyl)-(1→4)-O-(2,3-di-O-methyl-α-L-rhamnopyranosyl)-(1→9)-oxynonanoyl-bovine serum albumin (ND-O-BSA). The isolation, purification and revision of the structure of LAM of M. leprae and M. tuberculosis has been published (10). The product used here, ND-O-BSA, was suspended in carbonate-bicarbonate coating buffer (pH 9.6) and Tween 80 at a concentration of 0.1% by direct sonication for 20-30 sec with a 3-mm probe. The suspension was diluted to the required concentration with the same Tween 80 buffer and 50 μl was added to the wells of polystyrene microtiter plates which were then incubated at 37ºC for 14-16 hr in a moist chamber. The wells were washed with phosphate buffered saline (PBS), blocked by the addition of 100 μl of PBS containing 5% bovine serum albumin (BSA), and incubated at 37ºC for 1 hr. The contents were aspirated, and 50 μlof human serum diluted with PBS containing 20% normal goat serum (PBS-NGS) was added. The plates were incubated at 37ºC for 1 hr and washed with PBS followed by the addition of peroxidase-conjugated goat anti-human immunoglobulin M (IgM) (Cappel Laboratories, Downington, Pennsylvania, U.S.A.) diluted 1:1000 in PBS-NGS. After a 1-hr incubation and five further washings, 50 μl of H2O2-O-phenylenediamine substrate-dye reagent in citrate phosphate buffer (17) was added. The plates were then incubated at 37ºC for 30 min. Reactions were terminated with 2.5 N H2SO4 after the 30-min incubation and the absorbance was read at 488 nm (A488) (4). For each serum specimen, the absorbance in triplicate wells with BSA only was subtracted from those in which ND-O-BSA was the antigen before an analysis was made. The working concentration of ND-O-BSA was 45 ng of rhamnose equivalent per ml of buffer.
Another ELISA protocol described by Voller, et al. (17) was employed with minor modifications when LAM was the solid-phase antigen. Briefly, LAM (1 μg/ml)was suspended in carbonate-bicarbonate buffer, pH 9.6, by brief sonication. Then, 50 μlof this solution was added to wells of microtiter plates. The plates were incubated overnight at 37ºC, washed with PBS, pH 7.4, containing 0.05% Tween 20 (PBST). A "blocking" solution composed of PBST containing 1.0% BSA (100 μl)was added to the wells, and the plates were incubated for 1 hr at 37ºC. After emptying the blocking solution, 50 μlof serum diluted (1:1000 for LAM) in PBST containing 10% NGS was added, and the plates were incubated for 1 hr at 37ºC. After washing, 50 μlof affinity-purified, peroxidase-conjugated goat anti-human IgG diluted 1:1000 in PBS-10% NGS was added to the wells, and the plates were incubated for 1 hr at 37ºC. Finally, after a further washing with PBST, 50 μl of the substrate solution, composed of citrate-phosphate buffer, pH 5.0, containing 0-phenylenediamine (400 μg/ml) and H2Oz (0.01%) was added to the wells and incubated in a dark chamber for 15 min at room temperature. After the reaction was stopped with 50 μl of H2SO4, the absorbance was read at 490 nm in a multiscan ELISA reader. Each serum specimen was tested in triplicate, and the absorbances obtained without antigen were subtracted from those with antigen before evaluation.
RESULTS
For the purposes of defining normal antibody levels we first evaluated the optical density (OD) of the sera from a healthy control population in Colorado (U.S.A.) and decided that seropositivity would be determined only at values greater than 3 standard deviations (S.D.) from the mean OD of that population. For these purposes the mean ND-O-BSA serum IgM OD was determined in 116 sera as 0.03 ± 0.02 S.D., and the mean LAM serum IgG OD was determined in 236 sera as 0.17 ± 0.08 S.D. Thus for ND-O-BSA an OD > 0.10 was considered elevated, and for LAM an OD > 0.40 was considered to be positive.
In conformity with previous studies in which the native glycolipid was mostly used, lepromatous patients (6 BL and 23 LL) in the first year of therapy were found to have significantly elevated antibody levels to both ND-O-BSA and LAM (Table 1). In these LL patients 21 of 23 (91%) patients had significant antibody to both antigens. In these patients the mean OD was 0.86 ± 0.55 for ND-O-BSA and 1.01 ± 0.43 for LAM. Also, in agreement with previous studies, it was less common to find positive antibody to either antigen in BT patients in the first year of treatment. Fourteen of 19 (74%) such patients had significant serum IgM antibody to ND-O-BSA but with a mean OD of only 0.40 ± 0.36. During this first year of treatment even fewer of these patients (7 of 19; 37%) had significant antibodies to LAM (mean OD 0.37 ± 0.32). Furthermore, in Table 1 it is noteworthy that the largest annual change in the average serum antibody to both antigens in both lepromatous and tuberculoid patients occurred in the first year of therapy.
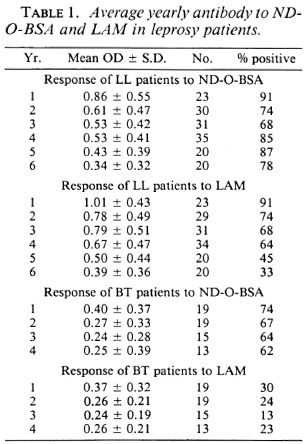
Table 2 details the average percent decrease in antibody/year for the first 5 years of treatment of all BT, BL and LL patients whose initial antibody level was found to be elevated. This suggests that for those BT patients who had positive antibody initially, the subsequent decline was similar to that seen in the LL patients. Overall it was most common for antibody levels to both antigens to fall gradually at < 20%/year as compared to more rapid interval rates of fall (20 %-40%, 40%-60%, etc.). The presence or absence of significant serum antibody to ND-O-BSA and LAM in all sera studied are summarized in Table 3. As can be seen, tuberculoid patients had antibody less commonly to both ND-O-BSA and LAM than lepromatous patients (p < 0.001). In all subjects when antibody was positive to only one of the two moieties it was usually to ND-O-BSA.


The change in antibody to ND-O-BSA and LAM with time in treated patients is quite variable (Fig. 1); while in some patients antibody to both antigens fell rather linearly and quickly (patients C, D, G, J), in others antibody levels remained relatively constant over several years (patients B, I, L). In still other patients, antibody levels fell slowly for a few years and often remained at a constant elevated positive plateau thereafter (patients A, C, E, F, H, I, K). While in most patients the absolute values and changes in antibody to the glycolipid (as measured with ND-O-BSA) roughly paralleled those of LAM (patients C, E, G, H, J, L), in others they were discrepant (patients A, B, D, F, I, K).
Fig. 1. Sequential serum antibodies (OD) in individual leprosy patients (A-L) to ND-O-BSA (

 ) and LAM (
) and LAM ( ---
--- ). Flat horizontal lines represent OD > 3 S.D. from the mean of a nonendemic population.
). Flat horizontal lines represent OD > 3 S.D. from the mean of a nonendemic population.
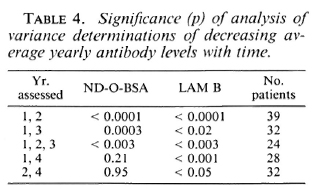
An analysis of variance of paired determinants was made on yearly mean antibodies in all patients who had one or more sera analyzed in a given year after the initiation of therapy (Table 4). These calculations were conducted for years 1,2, 1,3, 1,2,3,1,4, and 2 and 4. For both moieties there was a statistically significant drop in antibody in all three forms of leprosy between year 1, 2, 1, 3, 1, 2, 3(p < 0.02). The average yearly antibody responses to LAM fell significantly in all three forms of leprosy at all intervals studied. However, antibody to the phenolic glycolipid (ND-O-BSA) did not fall significantly when comparisons were made between years 1, 4 and years 2 and 4. Thus, a significant and consistent annual decrease in the antibody level to LAM could be demonstrated in the first 4 years of therapy, while antibody to the glycolipid (ND-O-BSA) decreased significantly only up to 3 years. Over the studied time intervals neither intercurrent type 1 or 2 lepra reactions appeared to materially alter the overall pattern of antibodies to cither moiety.
Two patients followed with sequential antibody levels relapsed during the course of this study (Fig. 2). In one of these lepromatous patients we observed a dramatic increase in antibody to the glycolipid (ND-O-BSA) and LAM just prior to the recognition of a clinical relapse. The other relapsed lepromatous patient had persistent, very high antibody levels. Such increases or lack of decline in antibody levels may indeed serve to alert clinicians to the possibility of noncompliance or resistant relapse.
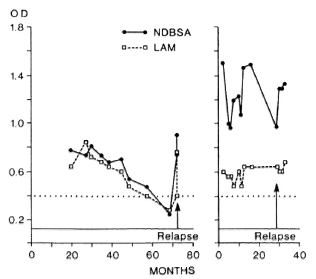
Fig. 2. Sequential serum antibodies (OD) in two relapsing lepromatous patients. Rat horizontal lines represent OD > 3 S.D. from the mean of a nonendemic population.
The antibody determinations of the 45 BL and LL San Francisco patients after an average of 10.5 years (6-35 years) of therapy, who were currently skin-smear negative, are of particular interest. Significant antibodies to the glycolipid (ND-O-BSA) and LAM were detected commonly: 19 (42%) had antibodies to both moieties; 11 (23%) to only ND-O-BSA; 6 (13%) to only LAM; while 9 (16%) had antibodies to neither. The average antibody as measured by OD in these patients was (0.31 ± 0.38) for ND-O-BSA and (0.57 ± 0.43) for LAM, and 0.43 ± 0.42 and 0.86 ± 0.36, respectively, for those who were seropositive. Thus, not only did many of these patients still have antibody to ND-O-BSA and LAM but they had rather high antibody levels.
Over the 10-14 years separating the collection of the paired sera for the Malaysian lepromatous patients, antibody levels to both the glycolipid (ND-O-BSA) and LAM fell significantly (p < 0.0005 for each). The OD for ND-O-BSA averaged initially 0.72 ± 0.55 and fell to 0.10 ± 0.14, while OD values for LAM on the first specimen averaged 0.94 ± 0.45 and on the second 0.31 ± 0.21. Over this time, antibody levels to the glycolipid (ND-O-BSA) fell in 25 of 26 patients, and antibody levels to LAM fell in 23 of 26 patients. However, of the 26 patients, 13 (50%) still had significant, albeit low, antibody levels-7 to ND-O-BSA (median OD 0.19) and 6 to LAM (median OD 0.67).
DISCUSSION
There is a paucity of objective means for following the progress of leprosy patients on chemotherapy. Clinical improvement is slow, variable and assessment is complicated by intervening reactional states which may materially affect the judgment of even the most experienced leprologist. M. leprae cannot be grown on artificial media or convincingly in tissue culture. Although M. leprae multiplies in the feet of mice, such rodent laboratories and animal facilities are not widely available. Furthermore, the model is sufficiently insensitive such that single doses of rifampin to previously untreated polar lepromatous patients regularly render M. leprae from their skin nonviable (14,15) and, paradoxically, after long periods of treatment some lepromatous patients still harbor in their tissues M. leprae which are viable in mice despite continued drug sensitivity (16,18,19). A traditional and worthwhile means of assessing the efficacy of therapy are skin smears which are generally taken from six disparate sites. Both the number of organisms present (bacterial index, BI) and their solid-staining characteristics (morphological index, MI) decline with effective therapy, the former generally becoming negative in about 5 years and the latter requiring only a few months or less. Skin smears are somewhat invasive and painful however.
At least at the lepromatous pole, the development of serologic tests for antibodies to M. leprae, and in particular those based on phenolic glycolipid, provided specific and sensitive diagnostic tools. It was hoped that following antibody responses might provide an objective means of evaluating individual responses to therapy. We previously found that lepromatous patients treated for 2 years or more had significantly lower levels of antibody to the glycolipid compared to those treated for less than 2 years and, with treatment, antibody levels generally decreased. This present study confirms that finding for antibody to LAM over the first 4 years and for antibody to the phenolic glycolipid for the first 3 years in all forms of leprosy. In general, in lepromatous patients undergoing initial therapy the BI (the log of the density of the number of M. leprae in the skin) begins at 3 + to 6 + and decreases one unit per year, thus usually decreasing at a rate equivalent to that of serum antibodies to the two major carbohydrate antigens found in these present studies. However, in evaluating individual patients, the rate of fall of antibody levels to these carbohydrate antigens is quite variable and, hence, could not likely be used to predict clinical response.
These studies also demonstrate that antibody to both the glycolipid (as measured using the neoantigen, ND-O-BSA) and LAM often persists at low levels for many years despite smear negativity. Thus, the continued presence of antibody does not indicate inadequate treatment. One could speculate that perhaps treated patients without significant antibody may be more adequately treated and comprise a subgroup of treated patients who are cured. However, at the state of our present knowledge the absence of significant antibody cannot be used as a measure of cure or to indicate that discontinuation of therapy is safe.
It is noteworthy that the Malaysian long-term treated, smear-negative patients had significant antibody to mycobacterial antigens less commonly than those in San Francisco (and at lower levels). This could be due to the Malaysian patients' duration of therapy being longer or, because of being institutionalized, they were more regular in their treatment.
Although they did not report bacteriologie status, Bach, et al. (1) also found that antibody to the phenolic glycolipid persisted in lepromatous patients (7 of 13) after more than 10 years of therapy. This tendency for serum antibody to the two dominant carbohydrate antigens of M. leprae, phenolic glycolipid and lipoarabinoman-nan, to remain despite effective therapy may be a function of the established fact that lepromatous patients generally harbor viable drug-sensitive M. leprae "persisters," even after many years of effective therapy and apparent cure (16,18,19). These persisters may be capable of continued stimulation of antibody responses. Phenolic glycolipid-I also may persist for long periods in the tissues, even in the absence of viable bacilli, both because of its insolubility and because of its relative indigestibility, thus promoting continued antibody formation. The persistence of antibody to LAM may be further explained either because it is of the IgG class and, hence, longer lived or because, being intimately associated with the cell wall (7), it may persist in the tissue long after bacterial demise. Another possibility is that since LAM is a common antigen among mycobacterial species, and indeed 8% of healthy individuals harbor significant IgG antibodies to LAM (9), leprosy patients may be repeatedly exposed to environmental mycobacteria, eliciting an anamnestic response.
Previously, we (5) and Brett, et al. (2) found a dramatic fall in IgM antibody to the phenolic glycolipid during erythema nodosum leprosum. The duration of this abrupt decrease in antibody appeared to last only several weeks, followed by a rebound. Thus, the fact that both we and Miller, et al. (12,13)found no overall difference in the pattern of changing antibody patterns in patients with reactional states is entirely consistent.
In conclusion, these studies confirm that untreated lepromatous leprosy patients and, less commonly, tuberculoid leprosy patients harbor anti-M. leprae serum antibodies to both M. leprae-spccificand nonspecific mycobacterial carbohydrate-based products. Importantly, antibody levels fall generally in the first few years of treatment. Thus, during the first several years of therapy consistently elevated levels of antibody that do not decrease, especially if directed to both moieties, could serve as a clue to the possibility of antimicrobial failure. This may be secondary to either noncompliance or drug resistance. In many areas of the world where mouse foot pad facilities are not available to monitor drug resistance, serial evaluations of serum antibodies could prove useful in monitoring treatment efficacy. The overall fall in antibody level docs not appear to be influenced by intervening reactional states. For both antigens, the initial quantity of serum antibody and the rate of fall of that antibody after the initiation of therapy vary considerably from patient to patient. The changes in antibody levels to both of these M. leprae products commonly parallel one another but are, at times, discrepant. Thus, the utility to the clinician of monitoring the actual rate of fall of serum antibodies appears limited. However, significant rises or persistently high serum antibody levels may be a harbinger of relapsed disease. Moreover, despite years of treatment and even after skin-smear negativity has been reached, significant antimycobacterial serum antibodies frequently persist.
Acknowledgments. This work was supported by contract 240-87-0037 from the GWL Hansen's Disease Center (to RHG) and contract No 1 AI-52582 (for PJP>) from the National Institute of Allergy and Infectious Diseases. We wish to thank Somphanh Phanivong for her substantial organizational assistance with this work.
REFERENCES
1. Bach, M-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
3. Chatterjee, D., Cho, S-N., Brennan, P. J. and Aspinall, G. O. Chemical synthesis and sero-reactivity of 0-(3,6-di-O-methyl-β-D-glucopyra-nosyl)-(l→4)-O-(2. 3-di-O-methyl-α-L-rhamno-pyranosyl)-( 1 -9)-oxynonanoyl-bovine serum albumin -the leprosy-specific , natural disacchar-ide-octyl-neoglycoprotein. Carbohydr. Res. 156(1986)39-56.
4. Cho, S-N., Fujiwara. T., Hunter, S. W., Rea, T. H., Gelber, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-β-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
5. Cho, S-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specific ity of phenolic ghcolipid-I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
6. Douglas, J. T., Steven, L. M., Fajardo, T., Cel-lona, R. V., Madarang, M. G., Abalos, R. M. and Steenberger, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
7. Gaylord, H. and Brennan, P. J. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Annu. Rev. Microbiol. 41(1987)645-675.
8. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacterid. 147 (1981) 728-735.
9. Hunter, G. W., Fujiwara, T. and Brennan, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
10. Hunter, S. W., Gaylord, H. and Brennan, P. J. Structure and activity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 261(1986)12345-12351.
11. Levis, W. R., Meeker, H. C, Schuller-Levis, G., Sersen, E., Brennan, P. J. and Fried, P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J. Infect. Dis. 156(1987)763-789.
12. Miller, R. A., Gorder, D. and Harnisch, J. P. Antibodies to phenolic glycolipid-I during long-term therapy: serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
13. Miller, R. A., Harnisch, J. P. and Buchanan, T. M. Antibodies to mycobacterial arabino-mannan in leprosy: correlation with reactonal states and variation during treatment. Int. J. Lepr. 52(1984)133-139.
14. Shepard, C. C., Levy. L. and Fasal. P. Rapid bactericidal effects of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21(1972)446-449.
15. Shepard, C. C. Levy, L. and Fasal, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1130.
16. Toman, K. Bacterial persistence in leprosy. Int. J. Lepr. 49(1981)205-217.
17. Voller, A., Bidwell. D. E. and Bartlett, A. Procedures, In: The Enzyme Linked Immunosor-bant Assay (ELISA). Alexandria, Virginia: Dy-natech Laboratories. Inc., 1979, pp. 23-125.
18. Waters, M. F. R., Rees, R. J. W., McDougall, A. C. and Waddell. A. G. M. Ten years of dapsone in lepromatous leprosy: clinical, bacteriological, and histological assessment and the finding of viable bacilli. Lepr. Rev. 45(1974)288-298.
19. Waters, M. F. R., Rees, R. J. W., Pearson, J. M. H., Laing, A. B. G., Helmy, H. S. and Gelber, R. H. Rifampin for lepromatous leprosy: nine years' experience. Br. Med. J. 1(1978)133-136.
20. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. M.D.; Kuzell Institute, San Francisco, California and GWL Hansen's Disease Center, Carville, Louisiana, U.S.A.
2. M.D., Kuzell Institute, San Francisco, California and GWL Hansen's Disease Center, Carville, Louisiana, U.S.A.
3. D.V.M., Ph.D., Colorado State University, Fort Collins, Colorado, U.S.A.
4. D.V.M., Ph.D., Colorado State University, Fort Collins, Colorado, U.S.A.
5. Ph.D., Colorado State University, Fort Collins, Colorado, U.S.A.
6. M.D., F.R.C.P., Director, National Leprosy Control Center, Sungai Buluh, Malaysia.
Reprint requests to Dr. R. H. Gelber, Kuzell Institute, 2200 Webster Street, San Francisco, California 94115, U.S.A.
Received for publication on 20 March 1989.
Accepted for publication in revised form on 5 June 1989.