- Volume 57 , Number 4
- Page: 752–65
Immunoepidemiological studies on subclinical infection among leprosy household contacts in Thailand
ABSTRACT
Three-thousand-fourteen leprosy household contacts in Thailand were surveyed by their personal history, physical examination, and immunological tests. The results were compared with those obtained f rom villagers in leprosy-endemic and nonen-demic areas. The percentages of young people, students, children and grandchildren of the patient, the contacts of multibacillary leprosy cases, long duration of contact, BCG vaccination, FLA-ABS and Dharmendra's lepromin-positive responders were significantly higher in the household contacts than those in the villagers. The percentages of neural and dermal symptoms were not significantly different between the household contacts and the villagers in the endemic area, but the percentages were higher than those of the villagers in the nonendemic area. A PPD skin test was more frequently negative in the former two groups than in the latter. Both FLA-ABS and lepromin tests showed a significant correlation with the age of the contacts, their occupations, blood relation to the patient, the duration of contact, BCG vaccination, dermal signs such as an ill-defined plaque or macule with or without sensory loss, but did not correlate with sex, type of leprosy in the patient, or other skin diseases. The FLA-ABS test in the household contacts and the villagers in an endemic area showed a significant correlation with the neural signs, such as enlargement of the peripheral nerve without sensory loss. These suspicious dermal and neural signs and symptoms were therefore considered signs of Mycobacterium leprae infection. The FLA-ABS test was sufficiently sensitive for detecting this infection and did not correlate with the lepromin or PPD skin tests. FLA-ABS-positive but lepromin-negative re-spondcrs were found in 33.5% of the household contacts. They were considered to be a high-risk group who may develop clinical leprosy. Nearly half of them were treated with dapsone or BCG according to the results of the PPD skin test. Follow up of these contacts, together with the remaining contacts without treatment, is in progress.RÉSUMÉ
On a étudié en Thailande 3.040 contacts domiciliaires de lèpre, en procédant à un interrogatoire concernant leur antécédents personnels, accompagné d'un examen physique et d'épreuves immunologiques. Les résultats ont été comparés avec ceux obtenus chez des villageois tant dans des régions endémiques pour la lèpre, que dans des régions non-endémiques. Par rapport aux villageois, les contacts domiciliaires, comprenaient une proportion plus élevée de personnes jeunes, d'étudiants, d'enfants et de petits-enfants de malades, de contacts de cas multibacillaircs, et de cas de longue durée. La proportion des personnes vaccinées par le BCG et de réponses positives aux épreuves d'anticorps fluorescents (FLA-ABS) et à la lépromine de Dharmendra était également significativement plus élevée chez les contacts que chez les villageois. La proportion de symptômes nerveux ou dermiques ne présentait pas de différence significative entre les contacts domiciliaires et villageois des zones endémiques, mais les pourcentages étaient cependant plus élevés que ceux des villageois habitant les régions non-endémiques. L'épreuve cutanée au PPD était plus fréquemment négative dans les deux groupes précédents que dans le dernier. Les épreuves à la lépromine de mêmes que celles au FLA-ABS, ont démontré une corrélation significative avec l'âge des contacts, leur occupation, leur relation de consanguinité avec le malade, la durée des contacts, la vaccination au BCG, les signes dermatologiques mal définis tels que plaques et macules avec ou sans perte de la sensibilité; il n'y avait cependant pas de corrélation avec le sexe, le type de lèpre ou la présence d'autres maladies cutanées. Les épreuves FLA-ABS chez les contacts domiciliaires de villageois ont montré une corrélation significative avec les signes neurologiques, comme les épaississements des nerfs périphériques sans perte de la sensibilité. Ces symptômes dermatologiques et neurologiques suspects ont été dès lors considérés comme des manifestations d'une infection par Mycobacterium leprae. L'épreuve FLA-ABS était suffisamment sensible pour déceler cette infection; elle ne présentait toutefois pas de corrélation avec les épreuves cutanées à la lépromine ou au PPD. Chez 33,5% des contacts domiciliaires, on a observé une réponse négative à la lépromine. Ceux-ci ont dès lors été considérés comme un groupe à haut risque, susceptible de développer une lèpre clinique. Environ la moitié d'entre eux ont soit été traités par la dapsone, ou bien ont reçu du BCG, selon les résultats de l'épreuve cutanée au PPD. On continue le suivi de ces contacts, de même que celui des témoins laissés sans traitment.RESUMEN
Se hizo un estudio de la historia personal, un examen físico y diversas pruebas inmunológicas, en 3014 contactos familiares de pacientes con lepra en Tailandia. Los resultados se compararon con los obtenidos en individuos habitantes de áreas endémicas y no endémicas. Los porcentajes de jóvenes, de estudiantes, de hijos y nietos de los pacientes, los contactos de casos de lepra multibacilar, el largo periodo de duración del contacto, la vacunación con BCG, y los respondedores positivos al FLA-ABS y a la lepromina de Dharamen-dra, fueron significativamente más altos en los contactos familiares que en la población no conviviente. Los porcentajes de síntomas neuralcs y dérmicos no fueron significativamente diferentes entre los convivientes y los no convivientes del área endémica pero fueron más altos que los encontrados en los habitantes del área no endémica. La reactividad dérmica al PPD fue más frecuentemente negativa en los primeros dos grupos que en el último. Tanto la prueba FLA-ABS como la prueba de la lepromina, mostraron una significante correlación con la edad de los contactos, sus ocupaciones, su relación sanguínea con los pacientes, la duración del contacto, la vacunación con BCG, y con signos dérmicos tales como placas o máculas mal definidas con o sin pérdida sensorial, pero no correlacionaron con el sexo, con el tipo de lepra en el paciente, o con otra enfermedad de la piel. La prueba FLA-ABS en los contactos convivientes y en los habitantes del área endémica mostraron una significante correlación con signos neuralcs tales como engrasamiento de los nervios periféricos sin pérdida sensorial. Estos sigos dérmicos y neuralcs sospechosos fueron considerados como signos de la infección con Mycobacterium leprae. La prueba FLA-ABS fue suficientemente sensible para detectar la infección pero no correlacionó con las pruebas dérmicas de la lepromina o PPD. Entre los contactos convivientes, el 33.5% de ellos fueron positivos para FLA-ABS pero negativos a la lepromina. Estos individuos se consideraron un grupo de alto riesgo que puede desarrollar la enfermedad clínica. Casi la mitad de ellos fueron tratados con dap-sona o BCG de acuerdo a los resultados en la prueba dérmica con PPD. El seguimiento de estos contactos y de aquellos sin tratamiento es un estudio en desarrollo.Subclinical infection in leprosy has recently been discussed as one of the most important problems from the epidemiological and immunological viewpoints (1,19,45). Bacteriological examination was first used by Figueredo and Desai (22) and Taylor, et al. (44) and immunological tests, such as the lepromin test and lymphocyte transformation test (LTT), have been used extensively by many investigators (9,27,37,39,41,47), but the disadvantage of these tests was their crossreactivity with other mycobacteria. The introduction of the indirect immunofluorescence test by Abe, et al. (3) was promising and desirable because the test could easily be rendered specific by absorbing the cross-reacting antibodies in the serum. An improved technique of this test, the fluorescent leprosy antibody absorption (FLA-ABS) test (4), has been used for detecting subclinical infection with Mycobacterium leprae in household contacts and school children (5,12,13). The serological sensitivity and specific ity of this test have also been confirmed by other investigators (7,11,26,33,46). Although some sera showed crossreactions with other mycobacteria, these reactions could easily be differentiated from a specific reaction to M. leprae by an additional absorption test (5,6). More recently, the technique of enzyme-linked immunoabsorbent assay (ELISA) has been used by several investigators (14,17,20,21,42,48). A major component of the phenolic glycolipid of M. leprae, PGL-I, isolated and characterized by Hunter, et al. (28,29), and a synthetic di- or trisaccharide epitope of this lipid have also been used as antigens for ELISAs (15,16,23-25,38) and the passive hemagglutination (PA) test (40). However, the ELISAs were found less sensitive, although more specific , than the FLA-ABS and PA tests for detecting subclinical infection in contacts (2). Most recently, the detection of antibodies against protein antigens of M. leprae has been attempted by some investigators (32,34). Using a M. leprae-specific monoclonal antibody competition assay, Sinha, et al. (43)found positive reaction in 46% of 28 contacts of multibacillary leprosy cases. Ash-worth, et al. (8) reported that only 6 out of 100 household contacts were positive in the same test.
Although the results of these tests have been very diverse, it is generally accepted that infection with M. leprae is far more common than is evidenced by cases of overt disease (45). Accordingly, the study of subclinical infection may be essential for understanding the epidemiology of leprosy infection. Another useful aspect of such studies is the possibility of prophylaxis of leprosy. In order to minimize new cases of leprosy, it is important to be able to detect subclinical infection as early as possible and to prevent immunodeficient individuals from developing overt leprosy by providing adequate treatment. Since the production of antibody to M. leprae does not necessarily reflect a deficiency of cell-mediated immunity (CMI) in leprosy, antibody studies must be complemented by the simultaneous use of any test for CMI. Therefore, the present study was planned and was initiated in 1980 with the following objectives and rationale: a) to measure the sensitivity and specific ity of the FLA-ABS test as a diagnostic tool to detect subclinical infection in leprosy, b) to identify asymptomatic individuals infected with M. leprae (i.e., seropositive) who are at high risk of developing multibacillary leprosy because of deficient CMI to M. leprae antigens, and c) to make a comparative study of the preventive efficacy of chemoprophylaxis and BCG vaccination among cases with subclinical leprosy. Results of the study concerning a) and b) are described in the present paper. The study on c) is still in progress.
MATERIALS AND METHODS
Study subjects
A total of 3014 household contacts in one leprosarium and seven leprosy colonies in Thailand were surveyed from 1980 to 1986. The number of contacts in each colony, the location of the colony, and the date of the first survey are as follows: 548 in Amnaj-Jaroen Colony (AJ), Ubol Province, August 1980; 762 in Maelao Colony (ML), Chian-grai Province, October 1981; 79 in Banhan Colony (BH), Mahasarakam Province, October 1981; 123 in Selapoom Colony (SP), Roi-ct Province, October 1981; 805 in Non Sompoon Leprosarium (NS), Khonkaen Province, November 1982; 353 in Prang-Kayang Colony (PK), Chandaburi Province, January 1985; and 239 in Pudhong Colony (PH), Nakorn-Srithammarat Province, January 1986. As a control group, 566 inhabitants of two villages -Non-Song Macw (NSM), Khonkaen Province, and Nong-Krapu (NKP), Petch-buri Province- and 605 in the other two villages-Non-kra-nuan (NKN), Khonkaen Province, and Ban-kokko (BKK), Supanburi Province -were surveyed in 1984.
These villages were classified by a prevalence rate (PR) of leprosy per 1000 population and categorized as follows: no patient (PR = 0) is nonendemic, PR < 1 is hypoendemic, PR 1-2 is mesoendemic, and PR > 2 is hyperendemic. In 1983, the rates in NSM and NKP were 20.0 and 16.0, respectively, while no patients were found in NKN or BKK in that year. All of the individuals underwent the following procedures.
Survey of personal history
A particular form was used throughout the study. The form consisted of name and code number, age, sex, address by code number, occupation, blood relationship with the patient, type (classification) of leprosy in the patient, and duration of contact by year. Since the number of specialists, administrators, office clerks, salesmen, skilled workers, security, communication and other service workers was not large, they were grouped into the category of businessmen. Family members with blood relations other than children and grandchildren were categorized as other. The types of leprosy were expressed by: MBL = multibacillary (lep-romatous and borderline) leprosy; PBL = paucibacillary (tuberculoid and indeterminate) leprosy. A contact of multiple cases of MBL and PBL in the household was classified as a contact of MBL.
Examination for clinical signs
Individuals with overt symptoms or signs of leprosy were excluded from the present study. The others were checked by the presence of a BCG scar, dermal and neural signs or symptoms, loss of sensation, and other skin diseases. Results of a BCG scar survey were recorded as: Yes = scar of previous BCG vaccination present; No = no scar and a negative history of having received BCG vaccination; Unknown = no scar and not quite sure whether BCG vaccination had been received. Those with a definite history of BCG but without a scar were considered to have received previous BCG vaccination. Palpable enlargement of peripheral nerves, muscle weakness, and anesthesia (loss of tactile and thermal sensations) were categorized as neural signs and symptoms. Dermal signs and symptoms consisted of infiltration, nodule, papule, plaque and macule. Those clinically diagnosed as of nonleprous origin were categorized as other skin diseases. Accordingly, the dermal signs and symptoms described below were mainly an ill-defined plaque or macule of unknown origin with or without loss of sensation.
Immunological tests
Lepromin test. Dharmendra's lepromin (18) was prepared by one of the authors (MA) at the National Institute for Leprosy Research (NILR), Tokyo, Japan. One-tenth ml of this lepromin was injected intracutaneously into a flexor side of the forearm, and the diameter of induration with redness was read after 48 hr. The result was expressed by a criterion recommended at the VII International Leprosy Congress, i.e., 0-4 mm is negative, 5-9 mm is doubtful, 10-14 mm is a weak positive (1+), 15-19 mm is a moderate positive (2+), and 20 mm or more is a strong positive (3+).
Tuberculin test. Purified protein derivative (PPD) was supplied by the Chest Hospital, Department of Communicable Diseases Control (CDC), Thailand, for the first survey and later by the Japan BCG Laboratory, Tokyo, Japan. The skin test with PPD (0.1 mg/dose) was simultaneously carried out in the contralateral forearm. Reading and grading of the reaction were the same as those in the lepromin test.
FLA-ABS test. Blood was collected by venipuncture, or finger prick in the case of some infants, before the injection of the skin-test reagents. The sera were kept on ice during the survey and subsequently kept in a deep freeze until use. Most of the sera were sent to NILR packed with dry ice. The technique of the FLA-ABS test (4) was slightly modified as described previously (2). The results of these immunological tests were read blind, i.e., without knowing any other data.
Chemoprophylactic and vaccination trials
Dapsone (DDS) was supplied by the Leprosy Division, Department of CDC, Thailand. BCG was supplied by the Japan BCG Laboratory, Tokyo, Japan. DDS was given daily according to body weight (1-2 mg/kg); BCG was given once a year. The treatment with either DDS or BCG was determined by the results of the immunological tests.
Statistical analysis
All of the data obtained by the above procedures from each individual were transcribed by marking a code card. The number of each mark on all cards was calculated by using an electronic statistical analyzer (PASKEY 1000; Gaikoku Bunken Co. Ltd., Tokyo, Japan). The chi-squared test was used for statistical evaluation. A probability value (p) less than 0.05 was considered to be significant. The Yates' correction was applied to the analysis if the actual number of individuals was less than 5 in any grouping. If this number appeared in a comparison of three or more items, the grouping of the individuals was modified so that the number of individuals in each group became 5 or more. Unknown and cases that were not done were omitted from the chi-squared test.
RESULTS
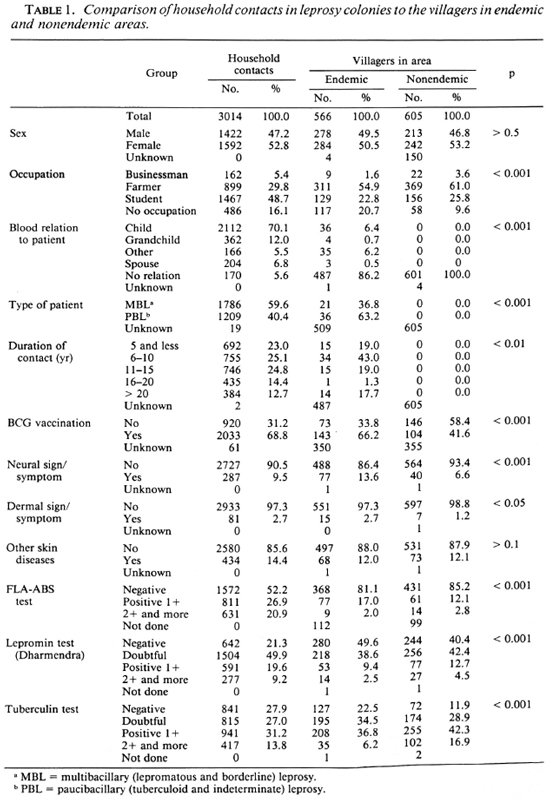
A frequency distribution of the ages of the household contacts was compared to those of the villagers in the leprosy-endemic and nonendemic areas. The results are shown in The Figure. It was apparent that a higher percentage of young people and a lower percentage of the aged were found in the household contacts in the leprosy colonies than in the villages. The numbers (and percentages) of the individuals classified by personal history, physical examination, and immunological test are shown in Table 1. The percentages of the respective groups in every item, excepting other skin diseases and sex, were significantly different among the household contacts and two groups of villagers. A higher percentage of the contacts than of the villagers was seen in students, children and grandchildren of the patient, contacts with MBL cases, long duration of contact, BCG vaccination, and FLA-ABS-and lepromin-positive responders. Percentages of both the neural and dermal symptoms and signs were not significantly different between the household contacts and the villagers in the endemic area, but the percentages in these two groups were higher than those in the villagers in the nonendemic area. Tuberculin-negative responders were less frequent in the villagers in the nonendemic area than in household contacts and villagers in the endemic area.
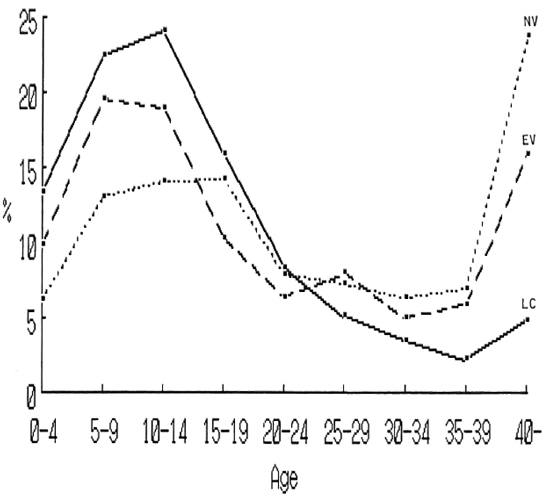
The Figure. Frequency distribution of the age of household contacts in leprosy colonics (LC) and those of villagers in endemic (EV) and nonendemic (NV) areas.
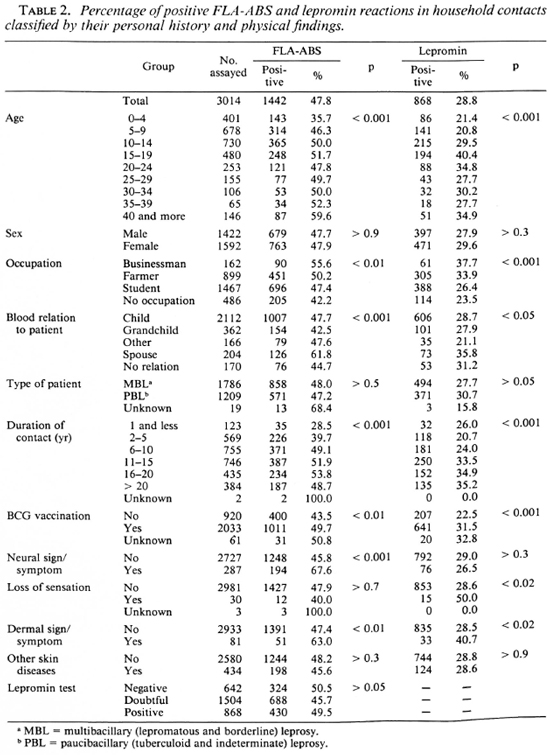
The results of the FLA-ABS and lepromin tests are shown in more detail in Table 2. The FLA-ABS test was less frequently positive in the 0-4 age group than in other age groups, while the lepromin test was less frequently positive in the 0-4 and 5-9 age groups than in the other age groups. Both tests were more frequently positive in contacts of a businessman or a farmer than in those without an occupation, in the spouse than those with blood relation to the patient, in those with a BCG scar than those without, and in those with a dermal sign or symptom than without. However, the percentage of positive reactions in both tests was not significantly different according to the type of leprosy of the patient. The FLA-ABS test was less frequently positive in those in whom the duration of contact was for 1 year or less than in those contacts with 6 years or more of contact. The lepromin test was less frequently positive in those with contact of 10 years or less than in those with longer contact. Neural signs or symptoms showed a significant correlation with the FLA-ABS test, but no correlation with the lepromin test. Loss of sensation showed a significant correlation with the lepromin test. No correlation was found between the two tests themselves. Enlargement of the auricular or ulnar nerve or both was the most frequent among the neural signs or symptoms. Ten cases with a plaque and 74 with a macule of unknown origin were found, apart from those with overt leprosy and those with other skin diseases. However, neither the FLA-ABS test nor the lepromin test was influenced significantly by the different localizations or different types of these suspicious signs and symptoms.
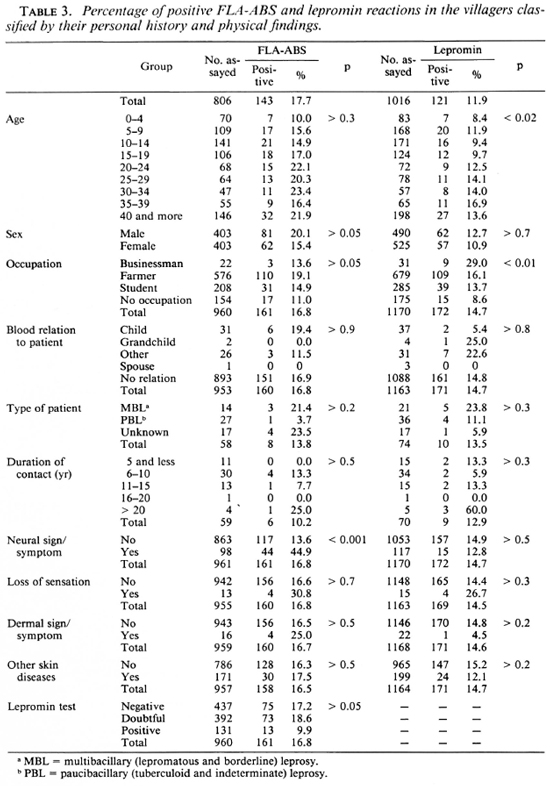
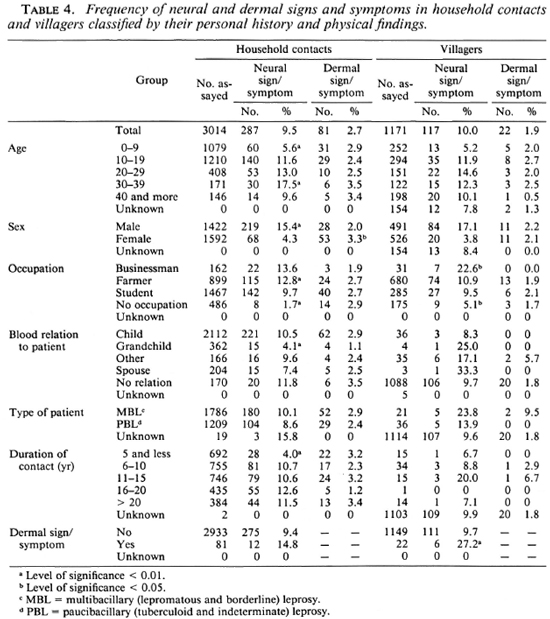
The results of these tests in the villagers are shown in Table 3. The FLA-ABS test showed a significant correlation with neural signs or symptoms only, while the lepromin test showed some differences according to age and occupation. The frequencies of the neural and dermal signs and symptoms were compared between household contacts and the villagers classified by their personal history and physical findings (Table 4). (The chi-squared value is omitted from the table and only a significantly higher or lower percentage is pointed out, as explained in the footnote.) The frequency of neural signs or symptoms in the household contacts showed a significant difference according to age, sex, occupation, blood relation to the patient, and duration of the contact. A different frequency of neural signs or symptoms was also found in the occupation of the villagers. On the other hand, dermal signs or symptoms of household contacts were more frequent in females than in males.
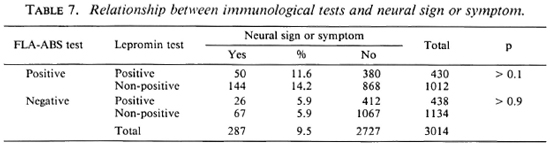
Tables 5-7 show the results of three-dimensional analyses on the household contacts. The nonpositive includes doubtful and negative reactions. The percentages of positive FLA-ABS tests were not significantly different according to the lepromin and tuberculin reactivities. However, the percentages of the positive lepromin tests were significantly higher in the tuberculin-positive responders compared with the nonpositive responders, irrespective of BCG vaccination. The neural signs or symptoms did not correlate with the lepromin test, although they did with the FLA-ABS test (Tables 2 and 7).
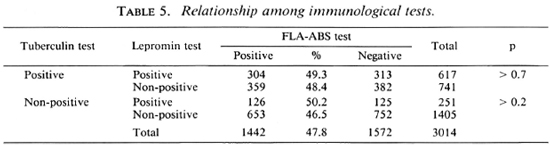

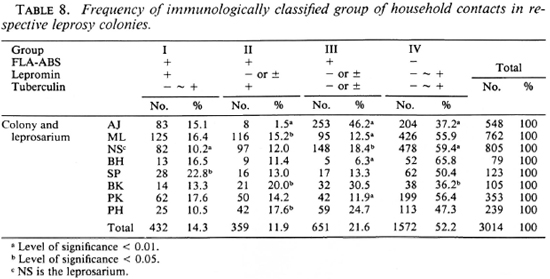
The household contacts in each leprosy colony were divided into four groups according to the results of the immunological tests (Table 8). The percentages of these groups were significantly different among the colonics. For example, group III was more frequent and groups II and IV were less frequent in the AJ colony than in the other colonies. Nearly half of the contacts in group II were treated with dapsone (DDS); those in group III, by BCG vaccination. The remainders in all of the groups were used as controls without treatment. All of them were followed up yearly, with a physical examination for symptoms and signs of leprosy, and lepromin and tuberculin tests; only in group II and group III was the FLA-ABS test repeated annually. The result of this follow-up study will be reported separately.
DISCUSSION
Although seven leprosy colonics and one leprosarium in different provinces of Thailand were under the leprosy control project, their populations and modes of living were not uniform and their cultural exchange with general society was relatively infrequent. These conditions made possible a broad epidemiological survey as well as longitudinal physical and immunological observations of the household contacts in each place. However, it was difficult to visit each place twice or more each year because the survey was conducted by investigators from two institutes in Thailand and Japan. Therefore, reading of Mitsuda's late reaction in the lepromin test had to be omitted. Dharmendra lepromin was also used because of its adaptability for reading the Fernandez reaction and its weak immunizing effect on the host after repeated testing (35,36).
The age distribution in the household contacts was significantly different from those in the villagers as the control groups.
This difference must be taken into account in interpreting the results of the FLA-ABS and lepromin tests, because the infants were found less responsive to both tests than were the older children and adults. In spite of this, the percentage of positive reactions in the total household contacts was significantly higher than that in the villagers. Moreover, the reactivity of both tests in the household contacts showed a significant correlation with their occupation and blood relation to the patient. These facts suggest that the immune responses to M. leprae are more strongly influenced by the physiological and living conditions than by the consanguinity with the patient. However, the type of leprosy in the patient did not correlate with the reactivity of either test. This result was in disagreement with the findings of other investigators (4,12,13,37,38,43), although the reason is not clear.
The percentages of positive FLA-ABS and lepromin tests in the villagers in the nonendemic area were 14.8% and 17.2%, respectively. Since they had no history of contact with leprosy, these positive reactions might be induced by either infection with M. leprae through the environment (30,31) or any species of other mycobacteria having antigenic similarity. Tuberculosis infection might be more frequent in these villagers than in the household contacts, as suggested by a different reactivity to PPD and its significant correlation with the lepromin test. Moreover, BCG vaccination had an accelerating effect on the reactivity of both the FLA-ABS and lepromin tests. In any case, the specific ities of these tests were considered too low for diagnostic use. On the other hand, the overdiagnosis of subclinical leprosy infection is a relatively small problem for the longitudinal observation of these individuals and for their treatment to prevent the disease. The sensitivity of these tests is more important than their specific ity for these purposes. The percentages of positive FLA-ABS tests in the household contacts showed a sufficient sensitivity and were comparable to those of the other tests (40,43) although significantly lower than those of the same test in a previous report (4). The percentages of positive lepromin tests in the household contacts were far lower than these values. Therefore, this test alone is useless for detecting subclinical infection with M. leprae.
A significant increase in the percentage of positive reactions with a) increasing age until adulthood and b) with the duration of contact < 20 years, occurred earlier in the FLA-ABS test than in the lepromin test. A similar finding was obtained in India (13). These results suggest an earlier induction of a humoral immune response to M. leprae than that of CMI after infection. A significant correlation between the FLA-ABS test and a neural sign or symptom such as the enlargement of the peripheral nerve without sensory loss was also found in a previous study (6). The lepromin test showed no correlation with the neural sign or symptom. It is therefore conceivable that the sign or symptom may be caused by the humoral immune response to M. leprae antigen released possibly from Schwann cells. An autoimmune response to nerve tissue antigen may also participate in the pathogenesis. These hypotheses may be proved by immunohistopathological studies if biopsy material can be obtained. On the other hand, dermal signs or symptoms such as an ill-defined plaque or a macule with or without sensory loss showed a significant correlation with both the FLA-ABS and lepromin tests. The dermal signs or symptoms may, therefore, be caused by both humoral and CMI responses, or predominantly the latter, in response to M. leprae in the skin. This possibility was endorsed by the independence of these tests from the other skin diseases.
Based on these considerations, the household contacts were divided into four groups with different immunological states. Group I is the contacts who have acquired both the humoral and CMI responses to M. leprae and therefore are at low risk of developing clinical leprosy. Group IV is those who have not been infected yet or have been infected with other mycobacteria or, although rarely, are unresponsive to M. leprae because of undeveloped or suppressed immunity. Groups II and III are those who have been infected with M. leprae without acquisition of CMI to this pathogen and, therefore, are at high risk of developing clinical leprosy. In fact, two cases with leprosy were found in these groups in Japanese contacts, and 33 out of 38 cases with leprosy belonged to one of these two groups in Indian contacts (10). Fourteen cases with leprosy were also found in our present study; their exact types of leprosy will be reported separately. It is therefore reasonable and rather desirable to treat the individuals in these two groups prophylactically. Since a killed M. leprae vaccine was not available at the time, BCG alone was used for the vaccination of half of those in group III. For a chemoprophy-lactic study, DDS was administered to half of those in group II who were tuberculin-positive responders. The results of these trials will be reported and discussed in the future.
Acknowledgments. This investigation received support from the Immunology of Leprosy (IMMLEP) component of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, the Japanese Leprosy Panel of the U.S.-Japan Cooperative Medical Science Program, and the Japan International Cooperation Agency (JICA). The authors express sincere thanks to all participants in the cooperative surveys in Thailand.
REFERENCES
1. Abe, M. Fundamental and practical requirements of serological test for detecting subclinical infection in leprosy. Proceedings of the Workshop on Serological Tests for Delecting Subclinical Infection in Leprosy. Tokyo: Sasakawa Memorial Health Foundation, 1983, pp. 5-18.
2. Abe, M., Bharadwaj, V. P., Buchanan, T. M., Douglas, J. T., Kim, D. I., Madarang, M., Petchclai, B., Ramu, G., Roscom, R. H., Sam-patavanich, S., Sampoonachot, P., Vithayasai, V., Wu, Q.-X. and Young, D. B. (International Cooperative Team for Evaluating Serological Tests in Leprosy). A trial to compare sérodiagnostic tests for leprosy. Tokyo: Sasakawa Memorial Health Foundation, 1988.
3. Abe, M., Izumi, S., Saito, T. and Mathur, S. K. Early serodiagnosis of leprosy by indirect immunofluorescence. Lepr. India 48(1976)272-276.
4. Abe, M., Minagawa, F., Yoshino, Y., Ozawa, T., Saikawa, K. and Saito, T. Fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
5. Abe, M., Miyaji, I., Okushita, T., Minagawa, F., Yoshino, Y., Sakamoto, Y. and Saikawa, K. Anti-mycobacterial antibodies in saliva. Lepr. Rev. 57 Suppl. 2(1986)213-223.
6. Abe, M., Ozawa, T., Minagawa, F. and Yoshino, Y. Subclinical infection in leprosy-its detection and control by fluorescent leprosy antibody absorption (FLA-ABS) test. Lepr. Rev. 52 Suppl. 1(1981)263-273.
7. Amezcua, M. E., Escobar-Gutierrez, A., Mayen, E. and Cazares, J. V. Sensitivity and specific ity of the FLA-ABS test for leprosy in Mexican populations. Int. J. Lepr. 55(1987)286-292.
8. Ashworth, M., Sinha, S., Patil, S. A., Ramu, G. and Sengupta, U. The detection of subclinical leprosy using a monoclonal antibody based radioimmunoassay. Lepr. Rev. 57(1986)237-242.
9. Balina, L. M., Fliess, E. L., Bachmann, A., Cardama, J. E. and Gatti, J. C. Similar alterations of lymphoblastic dedilferentiation in lepromatous leprosy patients and their healthy lepromin-neg-ative consanguineous oil-spring. Int. J. Lepr. 41(1973)7-13.
10. Bharadwaj, V. P., Katoch, K., Desikan, K. V., Mishra, B., Srivastava, A. K. and Yuhannan, A. Immuno-epidemiological studies using FLA-ABS test. Annual Report (1986-87), Central JALMA Institute for Leprosy, Agra, India, 43-47.
11. Bharadwaj, V. P., Ramu, G. and Desikan, K. V. Fluorescent leprosy antibody absorption (FLA-ABS) test for early serodiagnosis of leprosy. Lepr. India 53(1981)518-524.
12. Bharadwaj, V. P., Ramu, G. and Desikan, K. V. A preliminary report on subclinical infection in leprosy. Lepr. India 54(1982)220-227.
13. Bharadwaj, V. P., Ramu, G., Desikan, K. V. and Katoch, K. Extended studies on subclinical infection in leprosy. Indian J. Lepr. 56(1984)807-812.
14. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
15. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugatcs containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
16. Chanteau, S., Cartel, J.-L., Guidi, C, Pli-chart, R. and Bach, M.-A. Scrocpidemiological study on 724 household contacts of leprosy patients in French Polynesia using disaccharidc-oc-tyl-BSA as antigen. Int. J. Lepr. 55(1987)626-632.
17. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specific ity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
18. Dharmendra. The lepromin test-a review. Lepr. Rev. 18(1947)92-126.
19. Dharmendra. Detection of subclinical infection in leprosy. Lepr. India 54(1982)193-207.
20. Douglas, J. T., Celona, R. V., Abalos, R. M., Madarang, M. T. and Fajardo, T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Ccbu, The Philippines. Int. J. Lepr. 55(1987)718-721.
21. Douglas, J. T., Naka, S. O. and Lee, J. W. Development of an ELISA for detection of antibody in leprosy. Int. J. Lepr. 52(1984)19-25.
22. Figueredo, N. and Desai, S. D. Positive bacillary findings in the skin of contacts of leprosy patients. Int. J. Lepr. 18(1950)59-66.
23. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. C. Seroepidemiological studies of leprosy in Northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
24. Fujiwara, T., Hunter, S. W., Cho, S.-N., Aspin-all, G. O. and Brennan, P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharidc protein conjugate for serodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
25. Fujiwara, T. and Izumi, S. Synthesis of the neo-glycoconjugates of phenolic glycolipid-rclatcd trisaccharides for the serodiagnosis of leprosy. Agrie. Biol. Chcm. 51(1987)2539-2547.
26. Garraud, O., Ribierre, O. and Bach, M.-A. A follow up of T-cell subsets and of anti-.W. leprae antibody titer as measured by the FLA-ABS test in Melanesian leprosy patients under polychcmo-therapy. Int. J. Lepr. 54(1986)38-45.
27. Godal, T., Lofgren, M. and Negassi, K. Immune response to .1/. leprae of healthy leprosy contacts. Int. J. Lepr. 40(1972)243-250.
28. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
29. Hunter, S. W., Fujiwara, T. and Brennan, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
30. Kazda, J. Occurrence of non-cultivable acid-fast bacilli in the environment and their relationship to M. leprae. Lepr. Rev. 52Suppl.1(1981)85-91.
31. Kazda, J., Ganapati, R., Revankar, C, Buchanan, T. M., Young, D. B. and Irgens, L. M. Isolation of environment-derived Mycobacterium leprae from soil in Bombay. Lepr. Rev. 57 Suppl. 3(1986)201-208.
32. Klatser, P. R., de Wit, M. Y. L. and Kolk, A. H. j. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
33. Lee, H.-Y. and Kim, Y.-W. Study on diagnosis of leprosy by FLA-ABS test. Scicntia Lepro 4(1981)39-48.
34. Levis, W. R., Meeker, H. C, Shuller-Levis, G. B., Gillis, T. P., Marino, L. J., Jr. and Zabriskje, J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J. Clin. Microbiol. 24(1986)917-921.
35. Maeda, M., Matsumura, K. and Yoshida, K. The influence upon tuberculin reaction and Fernandez's reaction caused by the repeated intracutaneous injection of Dharmendra's antigen. 1st Report: The results in guinea pigs, injected repeatedly Dharmendra's antigen and dead BCG-suspension. Lepro 30(1961)69-75.
36. Maeda, M., Matsumura, K. and Yoshida, K. The influence upon tuberculin reaction and Fernandez's reaction caused by the repeated intracutaneous injection of Dharmendra's antigen. 2nd Report: Reinvestigation of the influence in BCG-vaccinated guinea pigs. Lepro 30(1961)76-80.
37. Menzel, S., Bjune, G. and Kronvall, G. Lymphocyte transformation test in healthy contacts of patients with leprosy. I. Influence of exposure to leprosy within a household. Int. J. Lepr. 47(1979)138-152.
38. Menzel, S., Harboe, M., Bergsvik, H. and Brennan, P. J. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy household contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
39. Nelson, K. E., Speck, S. M., Suprasert, S. and Smith, T. A study of cellular immunity in clinically healthy children of patients with leprosy in northern Thailand. Int. J. Lepr. 52(1984)147-153.
40. Petchclai, B., Khupulsup, K., Hiranras, S., Sampatavanich, S., Sampoonachot, P. and Lee-larusamee, A. A passive hemagglutination test for leprosy using a synthetic disaccharidc antigen. Int. J. Lepr. 56(1988)255-258.
41. Price, M. A., Anders, M., Anders, R. F., Russell, D. A. and Dennis, E. S. Cell-mediated immunologic status of healthy members of families with a history of leprosy. Int. J. Lepr. 43(1975)307-313.
42. Samuel, N. M. and Adiga, R. B. Detection of antibodies in sera of leprosy patients and contacts by enzyme linked immunoabsorbent assay (ELISA). Jpn. J. Lepr. 53(1984)32-37.
43. Sinha, S., Sengupta, U., Ramu, G. and Ivanyi, J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int. J. Lepr. 53(1985)33-38.
44. Taylor, C. E., Elliston, E. P. and Gideon, H. Asymptomatic infections in leprosy. Int. J. Lepr. 33(1965)716-727.
45. WHO Expert Committee on Leprosy. Sixth Report. WHO Technical Report Series No. 768, World Health Organization, Geneva (1988).
46. Wu, Q.-X., Ye, G.-Y., Ma, Z.-X., Qiang, N.-X., Liu, Q. and Zhou, L.-L. Preliminary application of fluorescent leprosy antibody absorption test. Proceedings of the Workshop on Serological Tests for Detecting Subclinical Infection in Leprosy. Tokyo: Sasakawa Memorial Health Foundation, 1983, pp. 25-28.
47. Yokota, T. Mitsuda reaction in children of leprous parents. Nagashima Kiyo 1(1954)4-8.
48. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. M.D., Leprosy Division, Ministry of Public Health, Thailand.
2. M.D., Raj-PrachaSamasai Institute, Leprosy Division, Ministry of Public Health, Thailand.
3. M.D., Heulah Land Services, Bangkok, Thailand.
4. M.D., Department of Communicable Disease Control, Ministry of Public Health, Thailand.
5. Khon-kaen University, Khonkaen, Thailand.
6. Khon-kaen University, Khonkaen, Thailand.
7. M.D., Kuriu Rakusen-en, Kusatsu-machi, Gunma-ken, Japan.
8. M.D., Tohoku Shinsei-en, Hasama-cho, Miyagi-ken, Japan.
9. M.D., Sasakawa Memorial Health Foundation, Sasakawa Memorial Hall, 3-12-12 Mita, Minato-ku, Tokyo 108, Japan.
Reprint requests to Dr. Abe.
Received for publication on 15 February 1989.
Accepted for publication in revised form on 20 June 1989.