- Volume 57 , Number 4
- Page: 766–76
Quantitative antibody ELISA for leprosy
ABSTRACT
Quantitative enzyme-linked immunosorbent assays (ELISAs) were established to measure IgM and IgG antibody levels to soluble Mycobacterium leprae sonicate (CD60) and to the synthetic disaccharide antigen based on the phenolic glycolipid-I antigen of M. leprae coupled to bovine serum albumin in 46 leprosy patients. Separate reference pools for IgM and IgG antibody were established. The reciprocal of the antibody titer was expressed as the number of arbitrary units in the reference pools which was subsequently used as the calibrator for assessment of units in individual test sera. The dose-response relationship for both IgM and IgG was highly specific and reproducible for both isotypes, as indicated by the intra- and inter-assay coefficients of variation. The distribution of antibody levels are in general agreement with the results f rom previous studies against different M. leprae antigens. The lepromatous group showed 10- to 100-fold higher IgM antibodies to both the soluble sonicate antigen and the disaccharide as compared to the control group. Very low to undetectable levels of IgM antibodies were observed in the tuberculoid group of leprosy patients. IgG antibodies, on the other hand, were not only present but showed considerable overlap with the lepromatous patient group.Optimized ELISAs, such as the one described in this study, would allow one to address issues such as antibody changes with treatment, antigen clearance, and correlation with other immune parameters associated with disease pathogenesis and protection.
RÉSUMÉ
Chez 46 malades, on a eu recours à des épreuves EL1SA pour mesurer les taux d'anticorps IgM et IgG à la fraction soluble du sonicat (CD60) et à l'antigène synthétique disaccharidique basé sur l'antigène phé-noglyco-lipidique-I de M. leprae couplé à l'albumine sérique de boeuf. On a établi des épreuves de référence séparées pour mesurer les taux d'anticorps IgM et IgG. La réciproque du taux d'anticorps a été exprimée en nombres d'unités arbitraires définies dans des groupes d'épreuves, nombres qui ont été reportés ensuite pour calibrer l'évaluation des unités dans les épreuves sé-riques individuelles. La relation entre la dose et la réponse tant pour les IgM que pour les IgG était hautement spécifique et reproductible pour les deux isotypes, ainsi qu'en témoignaient les coefficients de variation pour une même épreuve et entre épreuves différentes. La distribution des taux d'anticorps correspond en général avec les résultats obtenus lors d'études précédentes avec des antigènes de M. leprae. Lorsqu'on le comparait au groupe témoin, le groupe de malades lépromateux révélait des taux d'anticorps IgM 10 à 100 fois plus élevés tant contre l'antigène du sonicat soluble que contre l'antigène disaccharidique. Des taux très faibles ou même indécelables d'anticorps IgM ont été observés dans le groupe de malades testés. Par ailleurs, les anticorps IgG étaient non seulement présents, mais encore pouvaient recouvrir dans une mesure considérable les taux observés chez les malades lépromateux.Des épreuves ELISA améliorées, telles que celles décrites dans cette étude, permettent d'explorer des problèmes tels que les modifications d'anticorps au cours du traitement, l'élimination des antigènes, et la corrélation avec d'autres paramètres immunologiques et qui sont associés à la pathogenèse de la maladie et au pouvoir protecteur.
RESUMEN
Se desarrollaron ensayos inmunoenzimálicos (ELISAs) para medir los niveles de anticuerpos IgM c IgG contra un sonicado soluble de Mycobacterium leprae (CD60) y contra el disacárido antigenico del glicolípido fenólico-I acoplado a albúmina sérica bovina en 46 pacientes con lepra. Se prepararon mezclas de referencia para anticuerpos IgM e IgG. El valor recíproco del título de anticuerpo se expresó como el número de unidades arbitrarias en las mezclas de referencia y posteriormente se utilizó como calibrador para establecer las unidades en los sueros problema individuales. Como lo indicaron los coeficientes de variación intra- e ínter-ensayo, la relación dosis-respuesta para IgG c IgM fue altamente específica y reproduciblc. La distribución de los niveles de anticuerpo estuvo de acuerdo con resultados previos usando diferentes antígenos del M. leprae. El grupo lepromatoso mostró de 10 a 100 veces más anticuerpos IgM contra el antígeno sonicado soluble y contra el disacárido, que el grupo control. En los pacientes tuberculoides los niveles de anticuerpo IgM fueron muy bajos o no dctcctables pero los anticuerpos IgG, además de que estuvieron presentes, mostraron considerable sobreposición con el grupo de pacientes lepromatosos.Los ELISAs optimizados, tales como los descritos en este trabajo, permiten estudiar los cambios en los niveles de anticuerpos durante el tratamiento, la eliminación de antígeno, y hacer correlaciones entre estos parámetros y otros asociados a la patogénesis y a la protección.
Assessment of antibody levels in leprosy has several important applications, even though their role in terms of pathogenesis or protection is not defined. Potentially they are useful as indicators of contact with infection, subclinical infection, or overt clinical disease. Antibodies could therefore provide a useful tool for both the prevalence of contact with the disease and of clinical disease itself.
Antibodies to crude or partially purified leprosy antigens have shown a low level of specific ity (6,13). However, some of the purified antigens such as the phenolic glyco-lipid-I (PGL-I) have shown greater promise in terms of specific ity (1,15). With the application of gene-cloning techniques, highly purified antigens are becoming more and more available (21)- Most of the antibody assays have utilized radioactive (14), fluorescent (16), or enzyme-tagged probes (2) to detect antibodies for all isotypes (1,2). Of these, the enzyme-tagged probes have become the most popular due to their simplicity, ease of handling, and the potential for field application. A major limitation of these assays is their semiquantitative nature, making it difficult to evaluate the differences in populations as reported by different studies. Quantitative antibody assays utilizing similar standards could overcome this limitation and may provide greater insights into disease pathogenesis, protection, and incidence among individuals as well as among different populations.
Therefore, the aim of the present study was to establish a quantitative enzyme-linked immunosorbent assay (ELISA) which was optimized in terms of antigen coating and incubation times. High-titer reference pools were established and, finally, performance characteristics were established for determining different isotypes. Using our quantitative ELISA we were able to determine IgM and IgG antibodies to both Mycobacterium leprae soluble sonicate antigen (batch CD60) and synthetic disaccharide antigen coupled to bovine serum albumin (D-BSA) which is the immunogenic epitope of the PGL-I (8).
Both isotypes could be quantitated in a highly specific and reproducible manner as shown by inhibition with soluble antigen and by intra- and inter-assay coefficient of variation. The lepromatous group showed 10- to 100-fold higher IgM and IgG antibodies to soluble sonicate antigen as compared to the control group. The tuberculoid group, on the other hand, showed low to undetectable levels of IgM antibodies but high levels of IgG antibodies, overlapping to a certain extent with the lepromatous group. This system can now be modified for an array of antigens as well as for different isotypes and subclasses.
MATERIALS AND METHODS
Patient material. Forty-six untreated patients with histologically confirmed leprosy were studied. The study group included 25 lepromatous (BL/LL) and 21 tuberculoid (BT/TT) patients. The control group consisted of endemic healthy adults with no family history of leprosy. Five to ten ml of blood was collected in venoject tubes. Samples were kept at room temperature for 12 hr and then at 4ºC overnight before cen-trifugation in order to separate the serum. The sera were stored at - 70ºC in 0.8 ml aliquots until used.
Antigen. Both the armadillo-derived M. leprae-soluble sonicate antigen (batch CD60) and the synthetic disaccharide-BSA conjugated (D-BSA) (batch 10) were kindly provided by Dr. R. J. W. Rees, National Institute for Medical Research, London. The antigens were also aliquoted and stored at -70ºC until used.
Reagents. Bovine serum albumin (BSA) and the horseradish peroxidase (HRP) conjugated anti-human IgG and anti-human IgM (lot numbers 15F-8955 and 124F-8810, respectively) were purchased from Sigma, Poole, U.K. The substrate used was ortho-phenylenediaminc (OPD) (Sigma; lot number 54F-5003). Tween 20 was obtained from BDH Chemicals Ltd., Poole, U.K.
ELISA. Flat-bottom polystyrene plates (Flow Labs, Herts, U.K.) were used throughout the study. Briefly, 0.1 ml of the antigen was coated at 2 μg/mlin carbonate buffer (0.05 M, pH 9.6). The plates were washed three times with saline (0.15 M) containing 0.05% Tween 20. The remaining available sites were blocked with 0.2 ml of 5% BSA in 0.02 M phosphate buffered saline (PBS), pH 7.4, for 2 hr at 37ºC. The plates were then washed and incubated for a further 2 hr at 37ºC with 0.1 ml/well of appropriate dilutions of test sera in 2% BSA in PBS containing 0.05% Tween as the diluent. Reference sera were run at 9 twofold dilutions starting at 1/200 in duplicate. Test sera were run at six dilutions starting at either 1/500 for lepromatous sera or 1/50 for tuberculoid and control sera. The plates were then incubated with HRP-conjugated probes (anti-IgG or anti-IgM) at the manufacturers' recommended concentrations. The substrates used were OPD, prepared fresh immediately prior to use by adding 10 mg/ml OPD in absolute alcohol and 6% H2O2 to citrate buffer, pH 5, to give a final concentration of 0.1 mg/ml OPD and 0.003% H2O2. The reaction was stopped after 30 min with 4 M H2SO4, and the plates were read for optical density (OD) at 490 nm.
Inhibition of IgM and IgG antibodies with soluble antigen. To assess the specific ity of IgM and IgG binding, soluble antigen was used as the inhibitor. Equivalent volumes of varying amounts of soluble antigen were pre-incubated with the sera at 37ºC for 2 hr. Both reference and test sera were run at a single dilution representing the midpoint of the binding curve. These dilutions were 6400 for IgG reference serum and 3200 for IgM reference serum. Individual test sera ranged between 1600-3200. Identical serum dilutions with and without antigen incubation were used in the optimized ELISA described above. The only modification was that the plates were coated with 1 μg/ml of antigen. The plates were washed and developed in an identical fashion. Percent inhibition was calculated from net OD using the following equation:
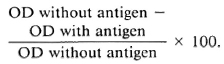
Statistics. Antibody levels in the different groups were compared by unpaired t tests and for different isotypes by paired t tests.
RESULTS
Reference sera pools
A reference serum pool was prepared using equal volumes of nine untreated lepromatous sera containing high titers of IgG and IgM antibodies. These sera were selected after screening them at two dilutions (100 and 1000) using a standard ELISA methodology (2). The minimum OD of individual sera at 1/1000 dilution was 0.6 for IgG and 0.4 for IgM pools. Leprosy-negative sera were similarly scanned at 1/50. A reference negative pool was prepared with nine sera giving less than 0.05 OD. These reference sera were then utilized for optimizing different assay parameters as well as for developing dose-response relationships.
Optimization of ELISA
Antigen concentration. To ensure antigen excess conditions, which is critical when developing a dose-response relationship using reference sera, the plates were coated with varying amounts (0.5, 1.0, 2.0 and 4.0 μg/ml) of soluble sonicate antigen CD60 in carbonate buffer, pH 9.6. The dose-response relationship is shown in Figure 1. The reference negative sera were run simultaneously and appropriate background readings were deducted. As is seen in Figure 1, even at an extremely high antibody concentration (1/200) no additional binding was observed by increasing the amount of antigen beyond 2 μg/ml. This pattern was consistent with both IgM (Panel A) and IgG (Panel B). We have, therefore, used 2 μg/ml of antigen to coat the plates for all further testing.
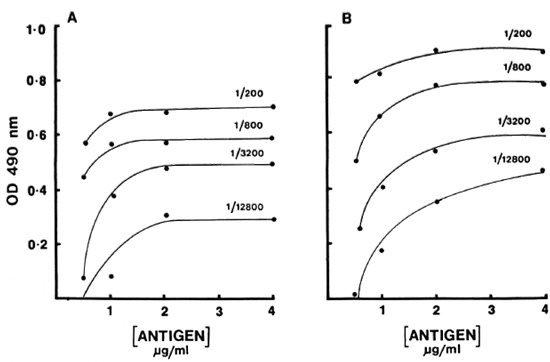
Fig. 1. Binding of IgM (A) and IgG (B) antibodies to increasing amounts of whole M. leprae sonicate antigens (CD60). A high titered serum was used at four different concentrations; results are expressed as OD490.
Serum binding. The effects of temperature and time on the antibody binding were also tested. Three different incubations (2 hr at 37ºC, 4 hr at 37ºC, and 4 hr at 37ºC followed by refrigeration overnight)-were used to develop the binding curve with reference pools (Fig. 2). Surprisingly, very little additional binding was observed after 37ºC for 2 hr. In fact, in the case of IgM antibody a slight decrease was observed. There was no effect on the sensitivity or the working range of the curve. The working range was approximately 1 log for both IgM and IgG antibody. Therefore, 2 hr at 37ºC was selected as the optimal time and temperature.
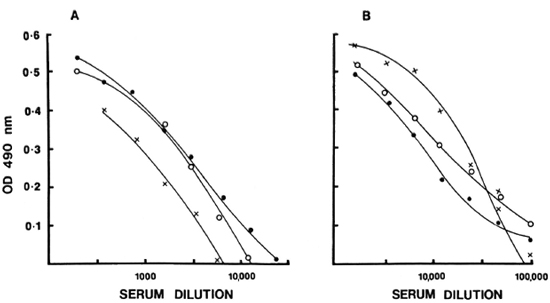
Fig. 2. Dilution curves of IgM (A) and IgG (B) antibodies to CD60 for varying incubations: (

 ) = 37ºC for 2 hr; (
) = 37ºC for 2 hr; (

 ) = 37ºC for 4 hr; (x
) = 37ºC for 4 hr; (x x) = 37ºC for 4 hr followed by 4ºC overnight. Results are expressed as OD490.
x) = 37ºC for 4 hr followed by 4ºC overnight. Results are expressed as OD490.
Standardization of reference pool
Full titration curves starting at 1/200 were developed for the reference sera using the optimal conditions indicated by previous tests. The titer was expressed as the highest dilution of serum resulting in an OD twice above the background binding obtained with the negative control serum pool at 1/200. The reciprocal of the titer was assigned as arbitrary units of activity in the serum. The units established in this fashion for IgG anti-M. leprae in reference serum was 51, 200 and for IgM, 6400. Subsequently, activity curves were generated with a minimum of seven values, and the results were expressed as OD obtained at different concentrations expressed as units for both IgG and IgM antibodies as shown in Figure 3. Individual test sera were read in the linear range of the curve for assignment of unit activity. To assess the specific ity and reproducibility of antibody binding in our assay system additional quality control studies were carried out to determine the calibration value of the reference pool.
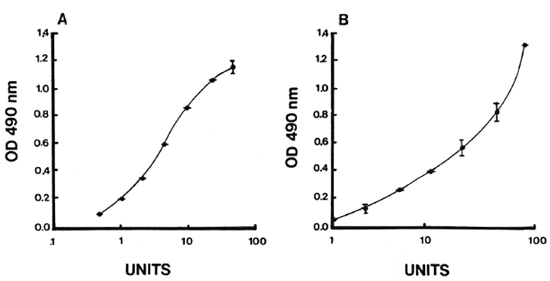
Fig. 3. Reference-dose-response curves for IgM (A) and IgG (B) anti-M. leprae were generated and expressed in terms of units assigned to each serum pool in relation to OD490. Mean standard error at each point from five independent assays obtained is indicated.
Quality control studies
Specificity studies. Binding of both IgG and IgM to solid-phase antigen in the presence and absence of soluble antigen as an inhibitor was studied with the reference pool as well as for four individual sera to determine the inherent specific ity of the assay system (Fig. 4). Eighty to 100% of IgM and IgG antibody binding could be inhibited with soluble antigen, indicating a high degree of specific ity within the system. Generally, a two- to threefold higher concentration was required for IgG to achieve the same level of inhibition as IgM, which may or may not indicate affinity differences.
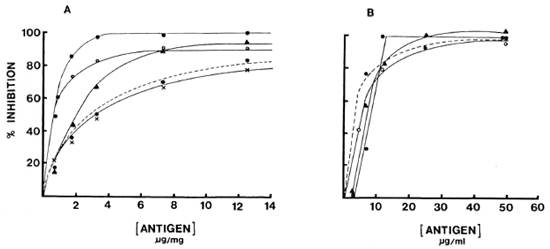
Fig. 4. Inhibition curves for reference pool (---) and four individuals sera (different symbols): A = IgM; B = IgG.
Intra- and inter-assay variation. Experiments were performed to evaluate the reproducibility and the precision of both the IgM and IgG anti-CD60 ELISA. The intraassay coefficient of variation (CV) was 2.4% (N = 5) for IgM anti-CD60 and 1.6% (N = 5) for IgG anti-CD60 (Table 1). The signal-to-noise ratio was approximately 10-fold for both IgG and IgM reference curves at the highest concentration, and the linear working range was over one log dilution (Fig. 5 A and B). Both parameters were, therefore, within acceptable limits. Inter-assay variation for three different sera (with low, medium and high antibody levels) run in three assays run independently are shown in Table 2. The inter-assay variation for each of the three sera for both IgM and IgG was less than 12%. Slightly less inter-assay variation was noted for sera with high levels of antibody than for sera with low levels of antibody, as expected due to interference with high concentrations of other serum factors.
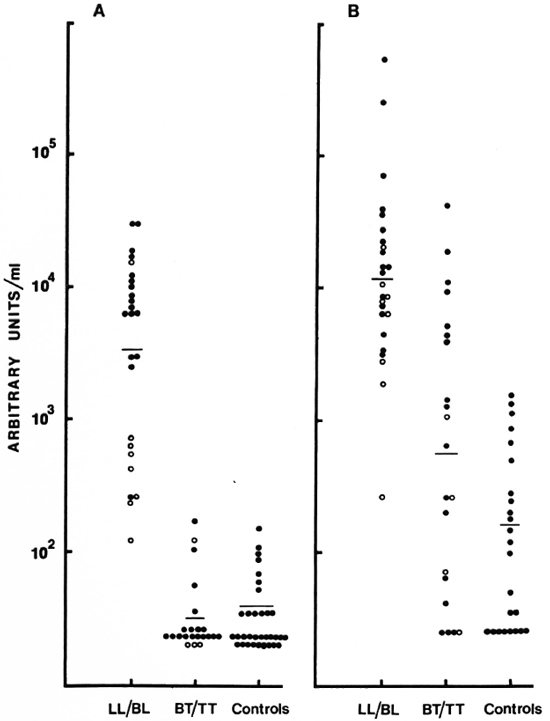
Fig. 5. Distribution of IgM (A) and IgG (B) antibodies to CD60. Each point represents one individual serum; geometric mean level indicated by horizontal bar. Results are expressed as units/ml obtained from a reference pool assigned arbitrary units. (![]() ) = LL and (
) = LL and (![]() ) = BL in the LL/BL group; (
) = BL in the LL/BL group; (![]() ) = BT and (
) = BT and (![]() ) = TT in the BT/ TT group.
) = TT in the BT/ TT group.
Quantitative assessment of IgG and IgM antibody
Quantitative assessment of IgM and IgG antibodies was carried out in 46 sera using the reference pools as the calibrators. The distribution of IgM and IgG anti-CD60 expressed as units/ml is shown in Figure 5. The two polar ends of the spectrum, LL/BL and BT/TT, were analyzed. Highly significant differences were observed between the LL/BL group and the control sera for the two isotypes (p < 0.001 for IgM, p < 0.05 for IgG). In contrast, the tuberculoid group showed low to undetectable levels of IgM which were not significantly different from the control group. This was not the case for the IgG isotype. Not only was the IgG level significantly higher than the control group (p < 0.05) but a much greater overlap was also seen with the lepromatous group (Fig. 5), suggesting a defective T-cell regulation of IgM antibodies in the lepromatous group but not in the tuberculoid group. There was also a suggestion of a bimodal distribution of IgM within the lepromatous group; the implication of such a distribution is not presently clear.
This assay was further modified to assess IgM levels to a purified antigen, D-BSA, which is the immunodominant determinant epitope of the PGL-I, a major component of the cell wall of M. leprae. Figure 6 shows the distribution of IgM to D-BSA. The pattern is identical to the one seen for the soluble sonicate antigen. There was no detectable level of IgG to D-BSA.
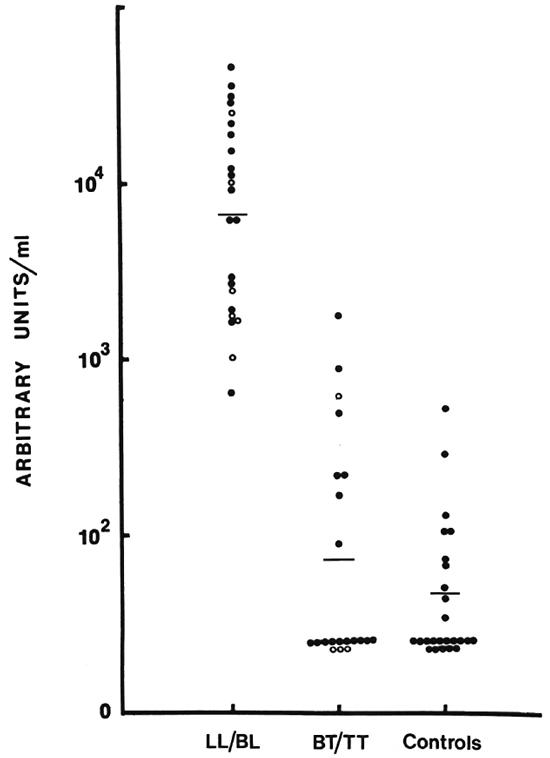
Fig. 6. Distribution of IgM antibodies to D-BSA (see legend to Fig. 5 for details).
DISCUSSION
ELISAs have been established to detect antibodies to M. leprae antigens (4,9,19). Most of these assays express antibody activity in terms of optical density (OD) readings. A positive serum control is used, but often only in order to monitor the reaction. Results are generally not related to any particular reference value, which makes inter-assay comparisons difficult. In some reports, however, the OD reading is related to a particular reference serum (2), the value being expressed as a percent of reference activity. Most of these studies utilize an optical density cut off using control sera which may be nonendemic sera (5) or endemic sera (7), resulting in varying levels of assay sensitivity and making inter-laboratory comparisons difficult. Finally, in these assays all sera, including reference sera, are run at a single dilution. Although point readings have the definite advantage of allowing mass screening of sera for sero-epidemiological studies, they usually do not take into account the activity curve, with increasing chances of sera being read in the nonlinear range, thus not indicating true differences among individual sera.
Quantitation is essential to allow the assessment of change in antibody levels over a period of time, for example, in contacts or, as in the case of patients with treatment or during reactions, to relate serological responses with cellular activity and make possible a comparison of the patients' responses to individual M. leprae antigens. Once reference sera with assigned units become available, as has been done for other immunoglobulin systems by the World Health Organization (18), meaningful comparisons would then become possible among various study populations and groups. Furthermore, in other systems it has also been possible eventually to convert the units on a weight-per-volume basis such as the IgE antibody units, and this may also be possible for this system in the future.
It was considered essential to optimize the assay in terms of antigen and serum binding conditions. Antigen excess is needed to achieve maximum binding of any single isotype and to reduce competition with different isotypes directed to the same antigen, especially when using M. leprae-soluble sonicate antigens. An antigen excess and optimal antibody incubation conditions would also eliminate variation due to affinity differences by ensuring maximal antibody binding.
In a number of studies, BSA-coated wells are used as controls for nonspecific antibody binding, particularly in the case of IgM (4,19). A more rigorous test to assess the specific ity of antibody binding is to utilize inhibition assays using soluble antigen. Our results clearly demonstrate the high degree of specific ity of binding for both isotypes. It was also interesting to note that generally a much higher concentration of soluble antigen was required to achieve 80% inhibition for IgG (10 μg/ml) as opposed to IgM (4 μg/ml). This may indicate either a greater diversity of antigens being recognized by the IgG system which may be represented in the soluble sonicate antigen at varying levels, or it may be due to a greater proportion of high-affinity antibodies which are more difficult to inhibit by soluble antigen. Secondly, the level of inhibition also varied within individual sera (Fig. 4). This is in keeping with the observation that the affinity of the antibodies may vary among individuals. Furthermore, antigen load also affects antibody affinity over time, even within individuals (11). An optimized ELISA such as the one described in this study would allow one to address these issues in greater depth.
The distribution of antibody levels is in general agreement with the results from previous studies with different M. leprae antigens (3,9,17). The patterns of IgM antibody levels were identical for both the soluble sonicate antigen and D-BSA. This is surprising in view of the fact that the soluble sonicate antigen contains very little of the PGL-I which is solubilized and removed during the processing of the M. leprae for sonication (10).
IgG antibodies to D-BSA antigen in the majority of the sera were below the range of detection in the present ELISA, supporting earlier observations with the PGL-I where the antibody response has been shown to be primarily an IgM response (20).
IgM antibodies to D-BSA are most likely to be indicative of the antigenic/bacterial load since it is not only a surface antigen but also the immunodominant epitope of the cell wall (12). The case of handling and solubility of D-BSA in contrast to the PGL-I make it a particularly attractive antigen for use in an ELISA. Preliminary quantitation of IgM anti-D-BSA and the correlation with the bacterial index in untreated patients has shown promise in terms of early detection of bacterial multiplication (manuscript in preparation). These observations have the potential of being extended for use in monitoring the leprosy control program in Pakistan.
Acknowledgments. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Sincere thanks are expressed to Dr. C. Draper, Mr. P. Burgess, and Professor K. P. W. J. McAdam, London School of Hygiene and Tropical Medicine, for their valuable advice and to Dr. S. Lucas, University College Hospital, for histological classification of the patients. We would also like to thank Miss A. Kifayat, Aga Khan University, and the staff of the Marie Adelaide Leprosy Centre, Karachi, for all their help and assistance. Finally, we would like to thank Dr. R. J. W. Rees and Dr. M. J. Colston, National Institute for Medical Research, London, for their encouragement and for the supply of M. leprae antigens.
REFERENCES
1. Brennan, P. J. and Barrow, W. W. Evidence for species specific lipid antigens in Mycobacterium leprae. Int. J. Lepr. 48(1980)328-387.
2. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
3. Brett, S. J., Payne, S. N., Draper, P. and Gigg, R. Analysis of the major antigenic determinants of the characteristic phenolic glycolipid from Mycobacterium leprae. Clin. Exp. Immunol. 56(1984)89-96.
4. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid 1 in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-183.
5. Burgess, P. J., Fine, P. E., Ponnighaus, J. M. and Draper, C. Serological tests in leprosy. The sensitivity, specific ity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidemiol. Infect. 101(1988)159-171.
6. Chakrabarty, A. K., Maire, M. and Lambert, P. H. SDS-PAGE analysis of M. leprae protein antigens reacting with antibodies from the sera from lepromatous patients and infected armadillos. Clin. Exp. Immunol. 49(1982)523-531.
7. Chanteau, S., Cartel, J.-L., Roux, J., Plichart, R. and Bach, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid 1 in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-776.
8. Cho, S.-N., Fujiwara, T., Hunter, S. W., Rea, T. H., Gelber, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-β-D-glucopyranosyl epitope for the sero-diagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
9. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specific ity of phenolic glycolipid 1 from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
10. Draper, P. Purification of M. leprae. In: Report of the Fifth Meeting of the Scientific Working Group on the Immunology of Leprosy (IMMLEP). TDR/ IMMLEP-SWG(5)/80.3 Annex 4 (1980) p. 4.
11. Eisen, H. N. and Siskind, G. W. Variations in affinities of antibodies during the immune response. Biochemistry 3(1964)996-1008.
12. Fujiwara, T., Hunter, S. W., Cho, S.-N., Aspin-all, G. O. and Brennan, P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
13. Harboe, M. and Closs, O. A claim for myco-bacterium specific antigen. Int. J. Lepr. 49(1981)85-87.
14. Harboe, M., Closs, O., Bjune, G., Kronvall, G. and Axelson, N. H. Mycobacterium specific antibodies detected by radio immunoassay. Scand. J. Immunol. 7(1978)111-120.
15. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
16. Ji, B. H., Tang, Q. K., Li, Y. L., Chen, J. K., Zhang, J. L., Dong, L. W., Wang, C. M., Ma, J. J. and Ye, D. L. The sensitivity and specific ity of fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Lepr. Rev. 55(1984)327-335.
17. Melsom, R., Harboe, M., Myrvang, T., Godal, T. and Belehu, A. Immunoglobulin class specific antibodies to M. leprae in leprosy patients including the indeterminate group and healthy contacts as a step in the development of methods for serodiagnosis of leprosy. Clin. Exp. Immunol. 47(1982)225-233.
18. World Health Organization. Biological Substances, International Standards Reference Preparations and Reference Reagents. Geneva: WHO, 1984, p. 46.
19. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
20. Young, D. B., Dissanayake, S., Miller, R. A., Khanolkar, S. R. and Buchanan, T. M. Humans respond predominantly with IgM immunoglobulin to the species specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149(1984)870-873.
21. Young, R. A., Mehra, V., Sweetser, D., Buchanan, T., Clark-Curtiss, J., Davis, R. W. and Bloom, B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316(1985)450-452.
1. M.B.B.S, Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan.
2. Ph.D., Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan.
3. Ph.D., Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, London, U.K.
4. M.B.B.S., Marie Adelaide Leprosy Centre, Karachi, Pakistan.
Received for publication on 30 January 1989.
Accepted for publication in revised form on 13 June 1989.