- Volume 57 , Number 4
- Page: 777–87
Effect of preincubation on the proliferative response to antigen by cells f rom leprosy patients and healthy controls
ABSTRACT
One of the postulated mechanisms contributing to the selective T-cell hyporespon-siveness in patients with leprosy is receptor blockade, the characteristic feature of which is reversibility following preincubation of cells in vitro. To test this hypothesis, peripheral blood mononuclear cells (PBMC) obtained f rom 17 leprosy patients and 10 healthy Mantoux-positivc and -negative controls were cultured freshly or after a period of preincubation in serum-containing medium, and the proliferative responses to Mycobacterium leprae, BCG, and strepto-kinase-streptodornase (SKSD) were measured. On the basis of the response to M. leprae, the leprosy patients could be divided into low, intermediate and high responders. Preincubation for 2-16 hr resulted in enhanced proliferation by cells f rom moderate responders, but not f rom low or high responders. Although the effect was serum dependent, it was neither antigen specific nor was it confined to cells f rom leprosy patients. Thus, an increase in response to both the crossreactive and unrelated antigens BCG and SKSD occurred, and the same trend was observed when cells f rom healthy controls were preincubated in serum-containing medium. Furthermore, cells f rom different individuals displayed varying responses to different antigens following preincubation, suggesting that the effect of this step was neither confined to isolated individuals nor to particular antigens. The addition of pronase to the preincubation step did not further enhance the response to antigen over and above that obtained with preincubation alone. It was therefore concluded that the enhanced proliferative response to antigen following preincubation was an in vitro phenomenon dependent upon the culture conditions employed, was not specific to leprosy, and was not related to receptor blockade.RÉSUMÉ
Un des mécanismes évoqués pour expliquer en partie la réponse sélective insuffisante des cellules-T chez des malades atteints de la lèpre est le blocage des récepteurs, dont la manifestation caractéristique est la réversibilité après incubation in vitro préalable des cellules. Pour vérifier cette hypothèse, on a mesuré les réponses prolifératives à Mycobacterium leprae, au BCG, et à la streptokinase-streptodornase (SKSD), de cellules mononucléaires de sang périphérique (PBMC), obtenues chez 17 malades de la lèpre et chez des sujets témoins positifs ou négatifs pour l'épreuve de Mantoux. Ces essais ont été menés sur cultures fraîches, après une période d'incubation dans un milieu contenant du sérum. Sur la base de la réponse à M. leprae, les malades de la lèpre peuvent être classés en répondeurs faibles, intermédiaires ou élevés. L'incubation préalable pendant 2 à 16 heures a entraîné une prolifération accrue des cellules chez les répondeurs intermédiaires, mais non chez les répondeurs faibles ou élevés. Malgré la présence de sérum, la réponse n'était pas spécifique pour l'antigène; elle était de plus limitée aux cellules provenant de malades de la lèpre. On a enregistré un accroissement tant de la réponse aux antigènes croisés de M. leprae qu'aux antigènes non associés BCG et SKSD. Le même profil de réaction a été observé lorsque les cellules de témoins en bonne santé ont été incubées au préalable dans un milieu contenant du sérum. De plus, des cellules provenant de différents individus ont témoigné de variations dans la réponse aux différents antigènes. Ces observations suggèrent que l'effet de cette étape intermédiaire n'est pas limitée à des individus isolés, non plus qu'à des antigènes particuliers. L'addition de pronase lors de l'incubation préalable n'a pas entraîné une réponse aux antigènes dépassant celle qui était obtenue en utilisant l'incubation sans pronase préalable. On conclut dès lors de cette étude que l'accroisement de la réponse proliferative in vitro aux antigènes n'est pas en relation avec la lèpre, ni avec le blocage des récepteurs, puisqu'elle dépend des conditions de culture qui sont utilisées.RESUMEN
Uno de los mecanismos propuestos como contribuyentes a la hiporeactividad selectiva de las células T en los pacientes con lepra es el bloqueo de receptores cuya característica es la reversibilidad del fenómeno al preincubar las células in vitro. Para probar esta hipótesis, se midieron las respuestas proliferativas de las células mononucleares de sangre periférica (CMSP) de 17 pacientes con lepra y de 10 individuos sanos Man-toux-positivos y negativos, estimuladas con Mycobacterium leprae, BCG y estreptoquinasa-estreptodornasa (SKSD) antes o después de un periodo de incubación en medio con suero. En base a su respuesta hacia M. leprae, los pacientes con lepra pudieron dividirse en respondedores bajos, intermedios y altos. La prein-cubación durante 2-10 hs dió como resultado un aumento en la proliferación de las células de los respondedores moderados pero no de los respondedores bajos o altos. Aunque el efecto fue dependiente de suero, no fue ni específico de antígeno ni estuvo confinado a las células de los pacientes con lepra. Asi, ocurrió un incremento en la respuesta hacia antígenos tanto de reacción cruzada (BCG) como no relacionados (SKSD), y lo mismo se observó cuando las células de individuos sanos se preincubaron en medio con suero. Además, las células de diferentes individuos mostraron respuestas variables hacia diferentes antígenos después de la preincubación, sugiriendo que el efecto de ésto no estuvo limitado a individuos aislados ni a antígenos particulares. La adición de pronasa durante la preincubación no aumentó la respuesta hacia el antígeno por arriba de la obtenida sólo con la preincubación. Por lo tanto, se concluyó que aunque la incrementada respuesta proliferativa hacia un antígeno después de un periodo de prcincubación fue un fenómeno dependiente de las condiciones de cultivo empleadas, no fue específico de la lepra ni estuvo relacionado con el bloqueo de receptores.The mechanism underlying the selective reduction in T-cell responsiveness to Mycobacterium leprae antigens in leprosy patients is controversial. Various hypotheses have been put forward including interleu-kin-1 (IL-1) or IL-2 deficiency (11,23), a reduction IL-2 receptors (13), suppressor macrophages (18), a deficiency of antigen-specific T cells (21), and receptor blockade. The characteristic feature of the latter is its reversibility on preincubation of peripheral blood mononuclear cells (PBMC) in serum-containing medium (19) which is thought to be due to the removal of antigen-receptor complexes from the cell surface (15). Recently, it has been suggested that the cellular response to M. leprae in anergic lepromatous patients may be enhanced by incubation of PBMC in serum before exposure to antigen in culture (1,14). Reversibility of cellular anergy has also been described after preincubation of PBMC in serum in patients with chronic fungal infection (19), suggesting that receptor blockade may be a feature of chronic infection and may account for at least some of the anergy associated with that form of infection.
The present experiments were designed to explore the possibility that receptor blockade was operative in leprosy. Preincubation of PBMC in serum-containing medium before exposure to antigen resulted in an enhanced proliferative response on subsequent exposure to M. leprae antigens in vitro. The effect was serum dependent but was not antigen specific , and was observed with cells from both healthy subjects and leprosy patients.
MATERIALS AND METHODS
Antigens. Armadillo-derived lyophilized M. leprae bacilli (CD86) were kindly provided by Dr. R. J. W. Rees through the WHO/UNDP/World Bank Immunology of Leprosy program. M. bovis (bacillus Cal-mette-Guerin, BCG strain) was obtained from the Commonwealth Serum Laboratories, Melbourne, Australia. Freeze-dried bacilli were suspended in phosphate buffered saline, 0.15 M pH 7.2 (PBS) and sonicated for 15 min to yield M. leprae and BCG sonicates (5). The protein concentrations of the sonicates were determined with Folin's reagent and were adjusted to 1 mg/ ml before storage at -70ºC. Streptokinase-Streptodornase (SKSD) was purchased from Lederle Laboratories, Sydney, Australia. After reconstitution with sterile water, the solution was dialysed in 25-mm dialysis tubing (Union Carbide, Illinois, U.S.A.) for 48 hr against cold PBS, pH 7.4, to remove preservatives. It was then sterilized by passage through a 0.22-μm filter (Millipore, Bedford, Massachusetts, U.S.A.), and its protein concentration estimated using Coo-massie blue G-250 reagent (Pierce Chemical Company, Rockford, Illinois, U.S.A.) (4) before being stored at - 70ºC. Tuberculin purified protein derivative (PPD) was obtained from Statens Seruminstitut, Copenhagen, Denmark, at a concentration of 1 mg/ml and stored at 4ºC.
Concentrations of 10 μg/ml were found to be optimal on preliminary testing for all antigens and were used in all cultures.
Serum. Human A serum was obtained from a single donor at the Red Cross Blood Transfusion Service, Sydney, Australia. Fetal calf serum was purchased from Flow Laboratories, McLean, Virginia, U.S.A. The human serum was centrifuged at 4000 x g for 20 min to remove residual red cells and sediment, and then heat inactivated for 30 min at 56ºC before being stored in aliquots at -20ºC.
Patients. Venous blood was collected into preservative-free heparin 10 iu/ml (Fisons, Sydney, Australia) from 17 patients undergoing long-term treatment for leprosy at the leprosy clinic at Prince Henry Hospital, Sydney, Australia. The leprosy patients were classified according to the criteria described by Ridley and Jopling (16), and consisted of 8 patients with leproma-tous disease, 2 patients with borderline lep-romatous disease, 2 patients with borderline tuberculoid disease, and 5 patients with tuberculoid disease. Control subjects consisted of 4 nonexposed Mantoux-negative subjects (H1-4), 1 naturally Mantoux-positive individual (H5), and 5 healthy Man-toux-positive BCG vaccinees (H6-10).
Culture conditions. PBMC were isolated by centrifugation over Ficoll-Hypaque density gradients (13) within 4 hr of venisection followed by washing in RPMI 1640 (Flow Laboratories) containing 0.85 g/1 NaHC03, 25 mM HEPES, penicillin (50 mg/1) and streptomycin (100 mg/ml). After adjusting their concentration to 106 cells/ml in RPMI containing 20% human A serum, the cells were plated out in triplicate wells in flat-bottom microtiter plates (Flow Laboratories) at a final concentration of 2 x 105 cells in 200 μl per well. Twenty μlof antigen in RPMI (10 μg/ml) was added to triplicate wells and RPMI alone to control wells. The trays were incubated for 5 days at 37ºC in an atmosphere of 5% CO2 in air before each well was pulsed with 0.5 μCi of methyl 3H-thymidine (Amersham, England) in 25 μl sterile PBS. The cells were harvested 16 hr later onto glass filter discs (Titertek; Flow Laboratories, Sydney, Australia) with a cell harvester (Skatron, Liebyen, Norway). The discs were dried, transferred to vials, and 2.0 ml of 0.6 g% 2,5-diphenyl-oxazole in toluene was added. The 3H-thymidinc incorporation into newly synthesized DNA was measured in disintegrations per minute (dpm) by liquid scintillation spectroscopy with a 1218 RackBeta counter (LKB Wal-lac, Turku, Finland).
Preincubation. PBMC were suspended at a concentration of 106 cells/ml in RPMI 1640 containing 20% A serum in 5 ml Falcon 2063 cell-culture tubes (Becton. Dickinson & Co., Mountain View, California, U.S.A.) and incubated for 2, 16, 40 or 64 hr at 37ºC in an atmosphere of 5% CO2 in air. The preincubated cells were washed once in RPMI, and viability was determined by Trypan blue exclusion (10). The cells were then resuspended at a concentration of 106/ ml in RPMI with 20% human A serum and cultured with antigen as described above. PBMC from leprosy patients were cultured when fresh and after 2, 16, 40 and 64 hr of preincubation, while the control subjects were cultured freshly and after 16 hr preincubation only. Except where indicated, the sera used for preincubation were the same as that employed during cultures. Cell recovery and viability during preincubation were examined separately at various stages by incubating PBMC in triplicate culture tubes, under the conditions described above, so that a mean and standard deviation of recovered and viable cells could be calculated.
Analysis of cell phenotype. The pheno-type of the PBMC was analyzed when freshly isolated from venous blood, after extensive centrifugation and washing, and after preincubation. The following directly conjugated monoclonal antibodies were used: phycoerythrin conjugated (PE) LcuM3 (a macrophage/monocyte marker), and fluorescein conjugated (FITC) Leu3A (CD4) and Leu2A (CD8) (Becton, Dickinson). Cell aliquots of 106 were incubated with 1/50 dilutions of monoclonal antibodies in 1% bovine serum albumin (Pentex; Miles Diagnostics. Naperville, Illinois, U.S.A.) for 30 min at 4ºC. They were then washed twice in cold PBS, fixed in 1% paraformaldehyde in PBS, and analyzed on a FACS 440 fluorescent activated analyzer (Becton, Dickinson). Fluorescin and PE were excited by the 488 nm laser of an argon ion laser and the fluorescent emissions collected using 535 ± 15 nm and 575 ± 26 nm band pass filters, respectively. The percentage of monocytes present was also analyzed by nonspecific esterase staining (22) of triplicate smears of cultured PBMC, followed by counting of 500 cells per smear to derive a mean percentage and standard deviation of esterase-positive cells.
Pronase treatment of cells. Cells to be pronase treated were resuspended at a concentration of 5 x 106 /ml in serum-free RPMI. To 1 ml of cell suspension was added 1 ml of 1 mg/ml pronase solution (Calbio-chem, San Diego, California, U.S.A.) in RPMI. The mixture was incubated for 15 min at 37ºC, washed twice in cold RPMI, resuspended in RPMI with 20% human A serum, and preincubated at 37ºC in 5% CO2 in air overnight. Control cells were treated in an identical way without the addition of enzyme (24). Preincubated cells (with or without exposure to pronase) were then set up in culture with antigen as outlined above.
Analysis of results. Proliferative data (d DPM) were derived by subtracting the mean 3H-thymidine incorporation in triplicate wells containing medium alone from the mean for triplicate test wells containing antigen. A response was considered positive if the d DPM were greater than 2000 and the stimulation index (SI) was greater than 2 compared with control wells.
RESULTS
Proliferative response of PBMC from leprosy patients to M. leprae, BCG, and SKSD. Seventeen patients with treated leprosy were selected from across the clinical spectrum of the disease. The proliferative response of their PBMC to optimal amounts of sonicates of M. leprae and BCG and the unrelated antigen SKSD were measured in a standard five-day culture. The results were arranged in ascending order of magnitude of the response to M. leprae and, on this basis, the patients could be divided into three broad groups as shown in Figure 1. The first group (subjects 1-2 with lepromatous leprosy) were low (nil) responders; the second group (subjects 3-14) had an intermediate response (5000-60,000 d DPM); the third group (subjects 15-17) were high rcsponders (greater than 150,000 d DPM). When the responses of the three groups to BCG and SKSD were compared with those to M. leprae (Fig. 1, B and C) no obvious correlation with M. leprae respondcr status was observed, with the possible exception of subjects 7 and 12 who had an intermediate-to-low response to all three antigens. These findings confirmed that the proliferative assay was not preferentially detecting a response to crossreactive T-cell epitopes shared by M. leprae and BCG, and that most leprosy patients across the entire clinical spectrum had intact T-cell reactivity to other unrelated antigens.
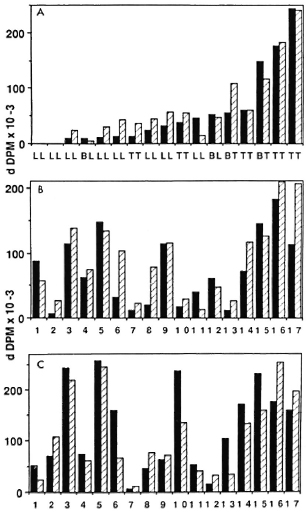
Fig. 1. Proliferative responses of PBMC from leprosy patients to M. leprae, BCG, and SKSD. Proliferative responses of PBMC to M. leprae (A), BCG (B) and SKSD (C) were assessed in 17 patients with treated leprosy using freshly isolated cells ( ) and cells which had previously undergone preincubation in serum-containing medium (
) and cells which had previously undergone preincubation in serum-containing medium (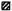 ). Clinical classification is shown in A; corresponding patient numbers in B and C (patients Ll-17).
). Clinical classification is shown in A; corresponding patient numbers in B and C (patients Ll-17).
Effect of preincubation of PBMC on the proliferative response to M. leprae, BCG, and SKSD. The mechanism underlying the selective reduction in T-cell reactivity of some leprosy patients to M. leprae antigens remains controversial. Among the various possibilities is receptor blockade by excess antigen, the characteristic feature of which is partial reversibility on preincubation in serum-containing medium (11). To test this hypothesis, PBMC from the same 17 patients were preincubated with medium containing 20% normal human serum for various lengths of time before being cultured in the presence of antigen. The cultures were set up in parallel with the non-preincubated controls described previously and their proliferative responses were compared (Fig. 1).
In approximately half the patients, preincubation for 2 to 16 hr led to an increase in the response to M. leprae sonicate. This phenomenon was not restricted to the relatively anergic lepromatous patients but was also seen in other patients across the leprosy spectrum. The effect was not antigen specific , since similar results were obtained when the other two antigens were tested. Finally, the preincubation response was not restricted to particular individuals, since an increase in response to some antigens but not others was shown in different subjects. In the remainder of the subjects, preincubation was associated with a decrease or no change in the proliferative response to M. leprae, BCG, and SKSD.
The increased response to M. leprae observed after preincubation for 16 hr appeared to be related to the level of the initial (control) response of fresh PBMC (Fig. 2 A). In other words, the great majority of subjects whose cells displayed this phenomenon had an initial response between 5000 and 60,000 d DPM and fell into the intermediate responder group (group 2). By contrast, preincubation did not affect cells from low and high responders (groups 1 and 3). A similar trend occurred on analysis of the influence of preincubation for the same period of time on the responses to BCG and SKSD (Fig. 2, B and C), although the cut ofT in d DPM below which preincubation had an effect was less evident.
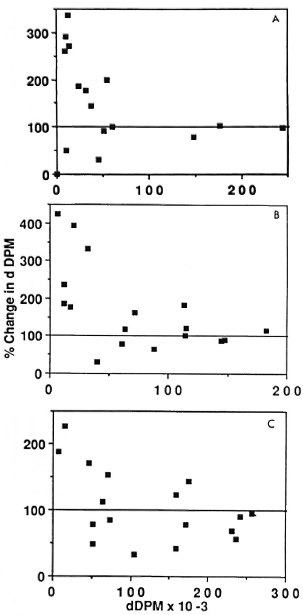
Fig. 2. Proliferative responses of PBMC from leprosy patients to antigen following preincubation is related to the fresh-cell response to antigen. In leprosy patients whose PBMC proliferated better to M. leprae following preincubation (A), the level of the initial fresh-cell response was in the intermediate range of 5000 to 60,000 d DPM. A similar but less striking pattern was evident for responses to BCG (B) and to SKSD (C).
When the proliferative response to antigen following preincubation was analyzed in terms of its duration, the length of preincubation required to elicit an optimum increase in the proliferative response lay between 2 and 16 hr for BCG and SKSD as well as for M. leprae. After 16 hr no effect on or a decrease in proliferation was observed (Fig. 3, A-C).
Fig. 3. Relationship between changes in proliferative responses of PBMC from leprosy patients to the duration of preincubation. Ratios of the preincubated response/fresh-cell response to M. leprae (A), BCG (B), and SKSD (C) are plotted as a function of the duration of preincubation in those leprosy patients who showed such a response. Most increases occurred after 2 hr or 16 hr; subsequently, the responses decreased.
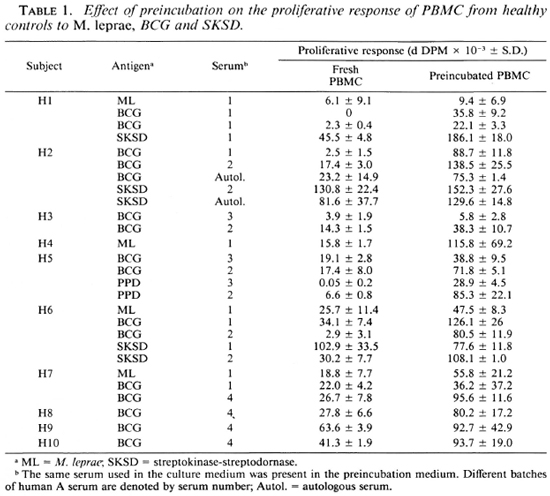
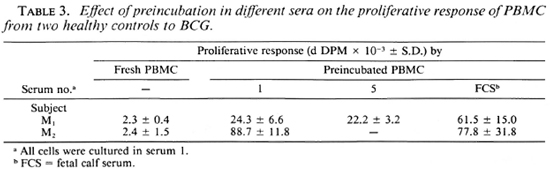
Effect of preincubation of PBMC from normal subjects on proliferative response. The lack of antigenic specifity of the preincubation effect suggests that T-cell anergy in leprosy cannot be explained in terms of receptor blockade, at least as measured by this technique. The findings, therefore, raised the question of whether the phenomenon was restricted to cells from leprosy patients. In order to test this possibility, cells were obtained from 10 healthy controls (including 4 Mantoux-negative and 6 Man-toux-positive donors) and were set up in culture as fresh cells or following preincubation for 16 hr in medium containing the same batch of human serum used in the previous experiments, autologous serum or other batches of human A serum. A comparison of the proliferative responses to PPD as well as to M. leprae, BCG, and SKSD revealed a significant increase following preincubation to one or more antigen in all 10 subjects (Fig. 4, Table 1). The results were reproducible, in that the same effect was observed upon repeating the experiment several times with cells from the same individuals using the same or a total of four different batches of human A serum. Thus, enhanced proliferation was not dependent on use of a particular batch of human serum although different sera were associated with a variable increase in response. Furthermore, replacement of human serum with fetal calf serum in the preincubation medium was also associated with an increased proliferative response to antigen (Table 3).
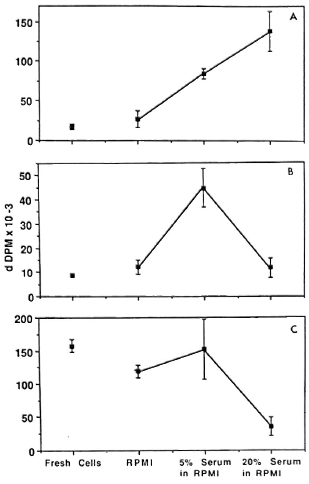
Fig. 4. Dose-response effect of serum in preincubation medium. Fresh-cell proliferative responses to BCG (A), PPD (B). and SKSD (C) were compared to responses following preincubation in medium without serum (RPMI 1640 alone) and with various concentrations of human A serum. A demonstrates a direct dose-response effect of serum to increase the response to antigen when the initial fresh-cell response was low. b demonstrates an initial increase which is not sustained when serum concentration is increased further. C demonstrates a lack of effect of serum when initial response is high.
Mechanism of the preincubation effect. These results suggest but do not prove that the preincubation effect required the presence of serum in the culture medium. PBMC from two normal donors studied previously (H3 and H5) were preincubated in RPMI alone or in the presence of RPMI containing a 5% or 20% concentration of one batch of human serum for 16 hr, and their responses to a range of antigens were compared with those of freshly isolated cells. Three representative profiles are illustrated in Figure 4 (A-C). The first indicates that proliferation increased following preincubation in serum-containing medium in a dose-depen-dent manner while the second shows an increase only in the presence of the 5% serum concentration. By contrast, in the third case the serum was inhibitory at the higher concentration. The difference between the two patterns was related to the level of the initial response of fresh cells, as had been demonstrated previously (Fig. 2). In other words, enhanced proliferation occurred with cells from intermediate but not high responders. The serum itself was not mitogenic, since the background counts did not increase after preincubation with either human serum concentration (data not shown).
This serum-dependent phenomenon could not be explained in terms of differences in cell recovery or viability induced by the presence of or absence of serum in the preincubation medium. Viability remained at or above 90% for up to 40 hr preincubation in the presence or absence of serum; whereas most of the cell loss occurred during washing and did not change significantly during preincubation (cell recovery after washing = 47.1 ± 15.4%; 2 hr preincubation = 47 ± 4.7%; 16 hr preincubation = 38.7 ± 5.3%; 40 hr preincubation = 41.1 ± 10.4%). In addition, the percentages of monocytes recovered after preincubation with or without serum, as assessed by FACS analysis, were comparable. For example, the proportion of monocytes in fresh cells and cells cultured for 2 hr and 16 hr in the absence of serum were 24.9%, 28.7% and 23.9%, respectively, and 24.1%, 27.3% and 23.1% when serum was present in the medium. These results were confirmed by nonspecific esterase staining (data not shown). Similarly, there was no alteration in the CD4:CD8 ratio with preincubation (2.2 for fresh cells and 2.1 after 16 hr preincubation).
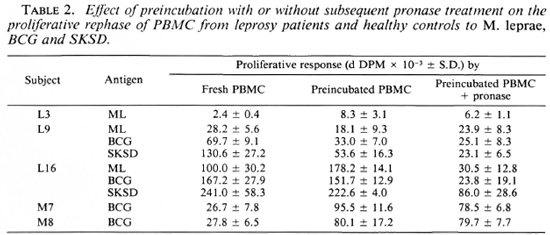
Effects of pronase on proliferative response of PBMC. It could be argued that preincubation was a suboptimal method for reversing receptor blockade, and that its combination with pronase treatment would be a more efficient way of removing cross-linked antigen or immune complexes. Three leprosy patients (L3, L9 and LI6) and two of the healthy controls (H7 and H8) were selected to test this possibility. Cells were cither washed and preincubated as described previously or treated for 15 min with pronase followed by washing and preincubation for 16 hr in medium containing 20% human serum. Recovery and viability were similar to that obtained in previous experiments. When the proliferative responses of the two populations of cells to the same antigens were compared, those which had undergone preincubation demonstrated an enhanced proliferative response to antigen, but no additional effect of pronase treatment over and above that of preincubation alone was observed (Table 2). Furthermore, the increase in response once again was not confined to cells from leprosy patients nor to M. leprae alone.
DISCUSSION
Mycobacterial antigen can be detected widely within the tissues of patients infected with M. leprae. M. leprae bacteremia has been described in lepromatous leprosy (9) and acid-fast bacilli are frequently identifiable in bone marrow, kidneys, and the liver as well as the main sites of infection in skin and nerves (2). M. leprae-specific antigens (such as phenolic glycolipid-I) may also be detected in serum and urine (7,12). The potential thus exists for antigen-induced receptor blockade to be operative as one of the mechanisms for the lack of protective immunity in leprosy, either system-ically or at the local neurocutaneous level. Indeed, treatment of some patients (with its attendant decrease in detectable antigen) has resulted in an improved proliferative response to M. leprae (6), suggesting that at least some of the anergy seen may be due to the presence of excess M. leprae antigen. In addition, murine models exist for the induction of tolerance by exposure to excess mycobacterial antigen (17). One cannot, however, exclude the possibility that the latter may be an artifact of studies using peripheral blood when reactive cells are sequestered in the cutaneous lesions (8).
The present experiments were designed to test the hypothesis that PBMC from leprosy patients anergic to M. leprae in proliferative assays would respond to this antigen after a period of preincubation in serum-containing medium. In line with previous work, the preincubation step was performed for both short (19) and long periods of time (14) so that a transient response would not be missed. Multiple antigens and sera as well as healthy controls were studied to determine the specific ity of any effect observed.
Preincubation of PBMC in serum caused an increase in the M. leprae proliferative response in approximately half of the leprosy patients studied. The increase was not restricted to the relatively anergic lepromatous subjects, but was seen in patients across the clinical spectrum. Furthermore, the effect of preincubation was restricted neither to specific individuals nor to M. leprae; similar changes were observed in response to both crossreactive (BCG) and non-crossreactive antigens (SKSD). Indeed, individuals could have different response patterns to different antigens (Table 1, Fig. 1). Patient 12, for example, responded to preincubation with no change (M. leprae), a decreased response (BCG), or an enhanced response (SKSD). The only factor which consistently influenced the outcome of preincubation appeared to be the level of the initial response by fresh cells: cells from intermediate responders usually proliferated better after preincubation; whereas the responses of low or high responders remained unchanged or were decreased (Fig. 2). While this relationship was most apparent for M. leprae, it also occurred with the other two antigens tested (Fig. 1). In fact, all experiments characterized by marked increases in d DPM after preincubation (e.g., doubling or tripling) were associated with an initial fresh cell proliferative response of less than 60,000 d DPM. Thus, when the appropriate controls were used, the effect of preincubation lacked specific ity for either cells from leprosy patients or for M. leprae antigen.
It is unclear why the serum-dependent increase in proliferation was not seen in the high responders or in all of the leprosy patients studied. For example, it is known that even cells from patients at the lepromatous end of the clinical spectrum can respond well to polyclonal activators such as con-canavalin A. Presumably, antigen stimulation is limited not only by the frequency of reactive cells but by regulatory influences as well. A clue to the occasional lack of increase in antigen responsiveness after preincubation may lie in the serum dose-response curves which indicated that preincubation in serum may upregulate a response to a certain limit, but no further (Fig. 4B). Alternatively, cells from high re-sponders may be already proliferating maximally to antigen regardless of the influence of serum (Fig. 4C). Finally, the batch of serum used may have influenced both the presence and magnitude of any response to preincubation (Table 3).
Although the reason for this serum-dependent phenomenon remains unclear, a clue to its mechanism is provided by the observation that the proliferative responses to antigen obtained when PBMC separated and cultured on the same day were influenced by the batch of serum used in culture (Table 1). Thus, the reason for the latter and for the variability in preincubation responses between batches of sera (Table 3) may be related to the presence in sera of varying concentrations of mitogenic or growth-promoting substances which, when coupled with preincubation, enhance the proliferative response when such cells are later exposed to antigen.
The dose-response effect of serum on the proliferative response was not due to changes in cell viability, yield or subset ratios, nor could it be explained by the alteration in culture duration from 6 days (fresh cells) to up to 8 days (preincubated cells) because optimal proliferation occurred after 6 days in culture (unpublished observations). In addition, there was no evidence of removal of potentially suppressive macrophages (18). Rather, the results suggest that preincubation in serum had induced a functional change in PBMC. Consistent with this conclusion was the short period of time (2 hours) required for the effect of preincubation to become apparent (Fig. 3) and its transient nature (2-16 hours). For the same reason death of CD8+ suppressor cells in the absence of antigen (14) seems to be an unlikely explanation. Furthermore, the phenomenon was not an artifact of the expression of results as d DPM; thus, subjects who displayed an enhanced response to antigen following preincubation had a parallel increase in their respective stimulation indices (data not shown). These findings make studies utilizing preincubation uninterprctable in the absence of adequate controls (14), and cast doubt on conclusions drawn on the role of CD8+ suppressor cells when such cells are deleted by complement lysis, a method which entails preincubation in serum (1).
Receptor blockade is characterized by the persistence of antigen on the cell surface due to saturation, crosslinking of antigen receptors and, perhaps, inhibition of re-expression of these receptors (15). While preincubation in medium alone has been shown to reverse this by removing excess antigen from the cell surface (19), pronase treatment was performed to enhance the chance of removing any residual antigen remaining after the preincubation procedure. Despite preincubation in serum overnight to allow time for the resynthesis of antigen receptors, pro-nase-treated cells displayed the same proliferative response to antigen as cells which had been preincubated alone. It was, therefore, concluded that the enhanced proliferative response to antigen following preincubation was an in vitro phenomenon dependent on the culture conditions employed, was not specific to leprosy, and could not be explained in terms of receptor blockade.
Acknowledgments. The project was supported by grants from the Lions Club (International) and the National Health and Medical Research Council (NHMRC) of Australia. One of the authors (RM) is in receipt of an NHMRC Medical Research Scholarship. The assistance of Mrs. M. Datt in preparation of the manuscript is greatly appreciated.
REFERENCES
1. Bach, M. A., Wallach, D., Flageul, B. and Cottenot, F. In vitro proliferative response to M. leprae and PPD of isolated T cell subsets from leprosy patients. Clin. Exp. Immunol. 52(1983)107-114.
2. Binford, C. H. and Connor, D. H., eds. Pathology of Tropical and Extraordinary Diseases, Volume 1. Washington, D.C.: Armed Forces Institute of Pathology, 1976.
3. Boyum, A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyte aggregating agents. Scand. J. Clin. Lab. Invest. 21 Suppl. 97(1968)31-50.
4. Bradford, M. A rapid and sensitive method for thc quantitation of microgram quantition of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72(1976)248-254.
5. Britton, W. J., Hellqvist, L., Basten, A. and Raison, R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
6. Bullock, W. E. Leprosy and other infections. In: Progress in Immunology II, Volume 5: Clinical Aspects. Amsterdam: North-Holland, 1974, pp. 193-202.
7. Cho, S-N, Hunter, S. W., Gelber, R. H., Rea, T. H. and Brennan, P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153(1986)560-569.
8. Desai, S. D., Birdi, T. J. and Antia, N. H. Presence of Mycobacterium leprae-reaclive lymphocytes in lymph nodes of lepromatous leprosy patients. Scand. J. Immunol. 28(1988)211-216.
9. Drutz, D. J., Chen, T. S. and Lu, W. H. The continuous bacteremia of lepromatous leprosy. N. Engl. J. Med. 287(1972)159-164.
10. Ford, W. L. and Hunt, S. V. The preparation and labelling of lymphocytes. In: Handbook of Experimental Immunology, Volume 2-Cellular Immunology. Weir, D. H., ed. Oxford: Blackwell Scientific Publications, 1973, p. 231.
11. Haregewoin, A., Godal, T., Mustafa, A. S., Be-lehu, A. and Yemanederhan, T. T-cell conditioned media reverse T-cell unresponsivess in lepromatous leprosy. Nature 303(1983)342-344.
12. Kaldany, R. R., Maasho, K., Ohman, R., Reitz-Vick, D., Britton, S. and Lefford, M. J. Methods for the detection of a specific Mycobacterium leprae antigen in the urine of leprosy patients. Scand. J. Immunol. 25(1987)37-43.
13. mohagheghpour. N., gelber, R. H., Larrick, J. W., Sasaki, D. T., Brennan, P. J. and Engle-man, E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleu-kin 2 receptors and is not reconstituted by inter-leukin 2. J. Immunol. 135(1985)1443-1449.
14. Mohagheghpour, N., Gelber, R. R. and Engle-man, E. G. T cell defect in lepromatous leprosy is reversible in vitro in the absence of exogenous growth factors. J. Immunol. 138(1987)570-574.
15. Nossal, G. J. V. and Layton, E. J. Antigen-induced aggregation and modulation of receptors of hapten-specific B lymphocytes. J. Exp. Med. 143(1976)51 1-528.
16.Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
17. Rook, G. A. W. The immunological consequences of antigen overload in experimental mycobacterial infections of mice. Clin. Exp. Immunol. 19(1975)167-177.
18. Salgame, P. R., Mahadevan, P. R. and Antia, N. H. Mechanism of immunosuppression in leprosy: presence of suppressor factor(s) from macrophages of lepromatous patients. Infect. Immun. 40(1983)1119-1126.
19. Schrader, J. W. and Nossal, G. J. V. Effector cell blockade; a new mechanism of immune hy-porcactivity induced by multivalent antigens. J. Exp. Med. 139(1974)1582-1598.
20. Stobo, J. D., Paul, S., Van Scoy, R. E. and Hermans, P. E. Suppressor thymus-derived lymphocytes in fungal infection. J. Clin. Invest. 57(1976)319-328.
21. Stoner, G. L., Mshana, R. N., Touw, J. and Be-lehu, A. Studies on the defect in cell-mediated immunity in lepromatous leprosy using HLA-D-identical siblings. Scand. J. Immunol. 15(1982)33-18.
22. Stuart, A. E., Mabeshan, J. A. and Davidson, A. E. Phagocytes in vitro. In: Cellular Immunology, Volume 2. 3rd cd. Weir, D. M., ed. Oxford: Blackwell Scientific Publications, 1978.
23. Watson, S., Bullock, W., Nelson, K., Schauf, V., Gelber, R. and Jacobson, R. Interleukin 1 production by peripheral blood mononuclear cells from leprosy patients. Infect. Immun. 43(1984)787-789.
24. Zola, H., Moore, H. A., Hunter, I. E. and Bradley, J. Analysis of chemical and biochemical properties of membrane molecules in situ by analytical flow cytometry with monoclonal antibodies. J. Immunol. Methods 74(1984)65-77.
1. M.B.B.S., D. Phil (Oxon), F.T.S., F.R.A.C.P., F.R.C.P., F.R.C.P.A., Clinical Immunology Research Centre, University of Sydney, Sydney, N.S.W., Australia.
2. A.O., M.B.B.S., D. Phil (Oxon), F.T.S., F.R.A.C.P., F.R.C.P., F.R.C.P.A., Clinical Immunology Research Centre, University of Sydney, Sydney, N.S.W., Australia.
Received for publication on 19 April 1989.
Accepted for publication on 23 June 1989.