- Volume 57 , Number 4
- Page: 788–93
A peptidoglycan protein complex purified f rom M. leprae cell walls contains most or all immunodominant M. leprae T-cell antigens
ABSTRACT
The outcome of an infection with Mycobacterium leprae is correlated with the T-cell-mediated immune response developed against this pathogenic agent. The identification of M. leprae antigens that are recognized by T cells is therefore of great importance. In this paper we present the results of in vitro lymphoproliferation assays in which T-cell reactivity was measured against a peptidoglycan-protein complex (PPC) which was purified f rom the cell wall of M. leprae. Twelve M. leprae-reactive T-cell clones with different antigen specific ities f rom a tuberculoid (TT) leprosy patient showed proliferative responses, but only when PPC was presented by HLA-DR-matched antigen-presenting cells (APCs). Four of these clones were known to react with the recombinant mycobacterial 65-kDa protein. A tetanus-toxoid-reactive T-cell line f rom a healthy control was not stimulated by this complex, supporting the idea that the stimulation by PPC was antigen specific . Both PPD-reactive and M. leprae-reactive T-cell lines f rom healthy individuals were stimulated by PPC. However, when this complex was presented to PPD-reactive T-cell lines derived f rom two lepromatous (LL) leprosy patients, we did not observe any proliferative responses. F rom these results we conclude that PPC contains most or all of the antigens which stimulate M. leprae-reactive T cells in association with relevant HLA class II molecules, including the 65-kDa protein or at least some immunogenic parts of it.RÉSUMÉ
L'évolution ultime d'une infection par Mycobacte-riiun leprae est étroitement associée à la réponse immunitaire que développent les ccllulcs-T de cet agent pathogène. L'identification des antigènes de M. leprae reconnus par les cellules-T est dès lors d'une grande importance. On présente ici les résultats d'épreuves in vitro sur la prolifération des lymphocytes, pour lesquelles la réactivité des ccllulcs-T a été mesurée en utilisant un complexe-protéine peptidoglycan-protéine (PPC) purifié à partir de la membrane cellulaire de M. leprae. Douze clones de cellules réagissant à M. leprae (TLC), mais ayant des spécificités antigéniques différentes, qui avaient été développés à partir de cellules obtenues d'un malade atteint de lèpre tuberculoide (TT), ont témoigné de réponses prolifératives, mais ceci uniquement lorsque le complexe PPC était présenté par des cellules APC assorties pour les antigènes tissulaires HLA-DR. Pour quatre de ces clones, on savait qu'ils réagissaient avec la protéine mycobactérienne recombinante 65-kDa. Ce complexe protéine ne stimulait pas l'anatoxine tétanique (TCL) obtenue chez un témoin en bonne santé. Ceci renforce l'hypothèse qui suppose que la stimulation par le PPC possède une spécificité d'antigène. Le PPC stimulait tant les cellules-T réagissant au PPD, que celles qui réagissaient à M. leprae, lorsque celles-ci étaient obtenues à partir d'individus en bonne santé. Néanmoins, lorsque ce complexe a été présenté aux lignées de cellules obtenues à partir de deux malades lépromateux (LL), aucune réponse pro-liférative n'a été observée. Ces résultats permettent de conclure que le complexe PPC-protéine contient la plupart ou même tous les antigènes qui stimulent les cellules-T qui réagissent à M. leprae en association avec les molécules HLA de classe II, pour autant qu'elles comprennent la protéine 65-kDa ou tout au moins certaines de ses composantes immunogéniques.RESUMEN
La evolución de la infección por el Mycobacterium leprae está relacionada con el desarrollo de una respuesta inmune específica mediada por células T. Por lo tanto, la identificación de los antígenos del M. leprae que son reconocidos por las células T resulta de gran importancia. En este trabajo se presentan los resultados de ensayos de linfoproliferación in vitro en los cuales se mide la reactividad de las células T contra un complejo de peptidoglicana-proteína (PPC) purificado a partir de la pared celular del M. leprae. Doce clonas de células T reactivas contra M. leprae (TLC), con diferentes especific idades antigénicas y derivadas de un paciente con lepra tuberculoide (TT), mostraron respuestas proliferativas sólo cuando el PPC fue presentado por células presentadoras de antígeno (APCs) portadoras del mismo HLA-DR. Se sabía que 4 de estas clonas reaccionaban con la proteína micobaetc-riana recombinante de 65 kDa. Una linea de células T reactivas al toxoide tetánico (TCL) derivada de un control sano no fue estimulada por este complejo. Esto apoyó la idea de que la estimulación por el PPC fue antígeno-específica. Tanto la TCL reactiva al PPD como la TCL reactiva al M. leprae (ambas derivadas de individuos sanos) fueron estimuladas por el PPC. Sin embargo, cuando este complejo fue presentado a las TCLs reactivas al PPD derivadas de 2 pacientes Ie-promatosos (LL) no se observó ninguna respuesta pro-liferativa. De estos resultados concluímos que el PPC contiene la mayoría (o todos) los antígenos (incluyendo a la proteína 65 kDa o al menos algunas de sus partes inmunogénicas) que estimulan las células T reactivas al M. leprae en asociación con las moléculas HLA clase II relevantes.Leprosy presents a continuous spectrum of clinical manifestations that closely parallel the T-cell-mediated immunity which is developed by the host against Mycobacterium leprae (1,14,16). The location and the nature of many antigens that are recognized by either helper- or suppressor-T cells, however, remain to be established. Recently five M. leprae proteins have been identified by monoclonal antibodies (6,21), three of which could stimulate a relatively small number of T-cell clones (TC clones) derived from leprosy patients (12,13,15). However, many M. leprae-reactiveT cells apparently do not recognize these proteins.
Several reports have indicated that the cell-wall skeletons of various mycobacteria induce cellular immune responses such as delayed-type hypersensitivity reactions in the skin (11). However, the antigenic properties of the cell wall of M. leprae have not been widely studied, although it is known that its peptidoglycan unit differs from those of other mycobacteria (4,5) and there is evidence for the existence of cell-wall-associated proteins (7) which might carry some T-cell epitopes.
Recently, Melancon-Kaplan, et al. (10)showed that purified cell walls stimulated proliferation of T cells from tuberculoid leprosy patients, and suggested that all or most of this activity was contained in a complex of peptidoglycan and proteins. We have systematically explored the possibility that this peptidoglycan-protein complex (PPC) purified from M. leprae cell walls contains important antigens involved in T-cell-mediat-ed immunity against M. leprae. To this purpose, we performed standard lympho-proliferation assays and presented this complex to different TC clones from a tuberculoid (TT) leprosy patient and T-cell lines (TC lines) obtained from patients with different types of leprosy as well as from healthy individuals, including leprosy contacts. Our results indicate that PPC contains all or more of the antigens that are recognized by M. leprae-reactiveproliferative T cells.
MATERIALS AND METHODS
TC clones. Peripheral blood mononuclear cells (PBMC) of a TT patient were isolated by Ficoll-Isopaque density centrif-ugation and restimulated with Dharmendra lepromin (1 μg/ml;Dr. R. C. Good, Centers for Disease Control, Atlanta, Georgia, U.S.A.) in Iscove's modified Dulbecco's medium (IMDM; Gibco, Grand Island, New York, U.S.A.) supplemented with streptomycin (100 μg/ml), penicillin (100 U/ml) (both How Laboratories, Scotland) and 10% heat-inactivated human serum (complete medium). The cultures were incubated for 5 days in 24-well tissue culture trays (Falcon 3047; Becton, Dickinson & Co., Oxnard, California, U.S.A.) at 37║C in a fully humidified CO2-air mixture. T-cell blasts were then enriched by Percoll density centrifugation, diluted to 5 blasts/ml in a feeder cell mixture consisting of 50 Gy irradiated autologous Epstein-Barr-transformed B cells (105 cells/ml), 30 Gy irradiated PBMC of 3-4 random donors (106 cells/ml), and Dharmendra lepromin (1 μg/ml), plated in 96-well flat-bottom microtiter plates (Falcon 3072; Becton, Dickinson) as 0.5 blast/well and incubated as described above. Growing cultures were transferred into 24-well tissue culture trays and restimulated with a feeder mixture supplemented with Leuko Agglutinin A (Pharmacia, Uppsala, Sweden). Three to five days later 10% interleukin-2 (IL-2) (Lympocault-T; Biotest, Federal Republic of Germany) was added to expand the clones. All clones used in this study had the phenotype CD3 + , CD4 + , CD8- and were restricted via DR2 and DR3 molecules. Four of them were previously described as "M. leprae-specific " while the others were crossreactive with two or more mycobacteria (15). Four of these TC clones were also known to react with the recombinant mycobacterial 65-kDa protein (20).
TC lines. PBMC of leprosy patients, healthy individuals, or leprosy contacts were restimulated with either Dharmendra lepromin (1 μg/ml), PPD (10 μg/ml; Statens Serum Institute, Denmark), or tetanus toxoid (1.5 Lf/ml; National Institute of Public Health, The Netherlands) as described above. On day 6, 10% IL-2 was added to expand the lines. After 7-10 days the cells were frozen at - 196║C in 1 ml ampules (Nunc, Denmark) containing 1-5 x 105 cells, 70% RPMI 1640 (Gibco), 20% pooled human serum, and 10% dimethylsulfoxide.
Antigens. Armadillo-derived M. leprae antigen was kindly provided by Dr. R. J. W. Rees, London, England. The peptido-glycan-protein complex (PPC) was purified from the cell walls of armadillo-derived M. leprae as described by Melancon-Kaplan, et al. (10), and was a kind gift of Dr. P. J. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A.
Proliferative assays. In complete medium in the presence of 0.2 ml antigen, 1 x 104 TC clones or TC lines and 5 x 104 40 Gy irradiated autologous or allogeneic PBMC as antigen-presenting cells (APC) were cultured together. The antigens tested were PPC (0.01-10 μg/ml)and, in some cases, PPD. PHA (1:200 dilution; Welcome Diagnostics, England), IL-2 (1:10 dilution; Biotest, Federal Republic of Germany), soluble M. leprae (1 μg/ml), tetanus toxoid (1.5 Lf/ml), and plain IMDM were used as controls. The cultures were set up in triplicate and incubated in conditions as described above for 88 hr. Sixteen hours before termination 1 μCiof [3H]-thymidine (Radiochemical Centre, England) was added to each culture. The samples were harvested on glass-fiber filters using a semi-automatic sample harvester. [3H]-Thymidine incorporation was assessed by liquid scintillation counting.
RESULTS
To study the T-cell reactivity induced by peptidoglycan-protein complex (PPC), we first selected 12 M. leprae-reactiveT-cell clones (TC clones) of a TT leprosy patient. The antigens that are recognized by eight of these TC clones were not known, while the other four were known to be reactive with the recombinant mycobacterial 65-kDa protein. In proliferation assays, PPC was presented at different concentrations to these TC clones. Over a concentration range of 0.1-10.0 μg/ml PPC, all of these TC clones showed a significant proliferative response (Table 1). The responses were comparable to that seen with whole M. leprae stimulation. However, when PPC was presented to some of these clones by antigen-presenting cells (APC) which were not HLA-DR matched, we did not observe any proliferation (Table 2). Furthermore, a tetanus-tox-oid-reactive T-cell line (TC line) of a healthy individual used in similar assays was not stimulated by PPC (Table 2).
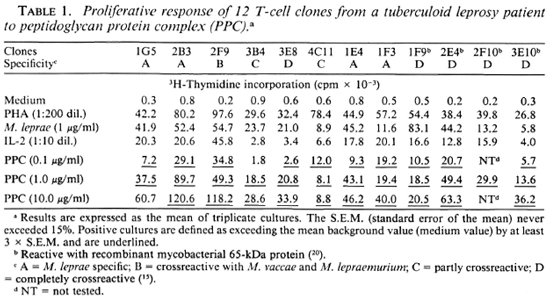
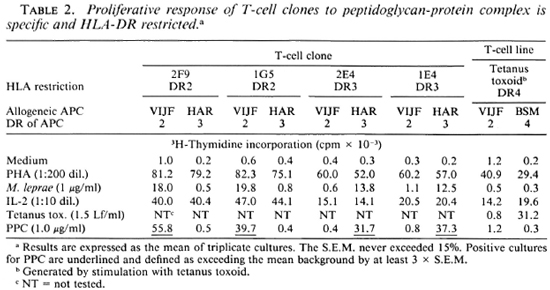
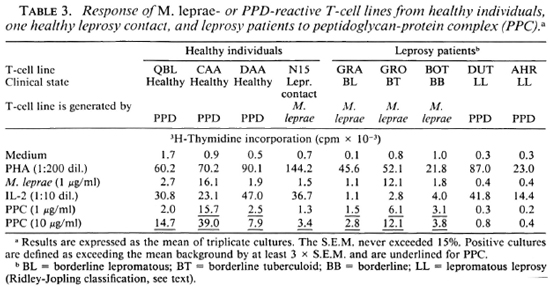
Further experiments were performed by using three TC lines from randomly selected healthy individuals generated by PPD stimulation and one TC line from a leprosy contact generated by M. leprae stimulation. The data presented in Table 3 show that all of these TC lines recognize PPC. Finally, PPC was presented to TC lines derived from different types of leprosy patients. According to the classification of Ridley and Jopling (16), 1 patient was diagnosed as borderline tuberculoid (BT), 1 as borderline leproma-tous (BL), 1 had midborderline (BB) leprosy, and 2 were polar lepromatous (LL) leprosy patients. The lines used were generated by either PPD or M. leprae stimulation. The proliferative responses of these lines to PPC are shown in Table 3. We observed that the lines from BL, BT and BB patients were stimulated by whole M. leprae as well as by PPC. However, the lines from the LL patients which were generated by PPD failed to demonstrate any proliferative response to either M. leprae or PPC. This indicates that at least the part(s) of the 65 kDa protein that contain the epitopes for these TLC are still present in PPC.
Recently, two papers were published in which the antigen reactivity of T-cell lines and clones raised with PPC was analyzed (8,9). The protein nature of the immunodominant cell-wall-associated antigens recognized by T cells was established, and these antigens were further defined using an im-munoblot technique (9). The 65-kDa heat-shock protein appeared to be present in cell wall preparations (8) and reactivity to a 65-kDa immunoblot fraction was observed (9). However, the data indicated that thus far unknown low molecular weight (7 kDa and 16 kDa) proteins might be the most immunogenic constituents of M. leprae cell walls (9).
DISCUSSION
In this study we have defined the T-cell antigenic characteristics of the peptidoglycan-protein complex (PPC) which was purified from the cell wall of M. leprae. There were mainly two reasons why we were interested in performing this study. First, the peptidoglycan of M. leprae differs from that of other mycobacteria in its chemical composition: glycine rather than L-alanine is found in the cross-linking tetrapeptide (4,5). This specific structure might play a role in M. leprae-specific immunosuppression observed in LL patients. The second reason was the presence of large amounts of protein (60.6%) in this complex. It is generally believed that the antigens that are recognized by T cells are proteins. Until now, five M. leprae proteins have been identified by monoclonal antibodies (6,21) but only three of them could stimulate a relatively small number of M. leprae-reactive TC clones derived from leprosy patients (12,13,15). The antigens that are recognized by many TC clones remain unknown. Thus, some other antigenic molecules, probably proteins, carrying important epitopes must be present in M. leprae. The identification or at least localization of these structures is essential to understand the factor(s) playing role(s) in either protective immunity against, or immunopathology induced by, M. leprae.
To explore the possibility that PPC might contain important T-cell epitopes, we performed in vitro lymphoproliferation assays in which PPC was presented to 12 carefully selected M. leprae TCclones of a TT leprosy patient and cither M. leprae- or PPD-reactive TC lines from healthy individuals and leprosy patients. We observed that all TC clones and TC lines from healthy individuals and M. leprae-reactive TC lines from BL, BT and BB patients were stimulated by PPC, while PPD-reactive TC lines from two LL patients did not show any proliferative response to this complex.
Since a large variety of peptidoglycan preparations, including peptidoglycans from some mycobacteria, are known to act as mitogens (17,19), we checked whether the strong T-cell stimulatory effect of M. leprae PPC observed by us might be due to a mitogenic effect on T cells. To this purpose, PPC was presented to some of the TC clones mentioned above by APCs which were not carrying relevant HLA class II molecules, and to a tetanus-toxoid-reactive TC line from a healthy individual. However, none of the T-cell APC-combinations which were not reactive with M. leprae were stimulated by PPC. Thus, our first conclusion is that PPC does not have any mitogenic effect on T cells in vitro. Therefore, we also conclude that this M. leprae cell wall PPC contains most if not all of the immunodominant T-cell epitopes of M. leprae, since this complex, in association with HLA class II molecules, stimulated all M. leprae-reactive TC clones used in this study. Some of these epitopes are M. leprae-specific ,because four of the TC clones used in this study were previously defined to react only with M. leprae. The M. leprae cell wall PPC also contains cross-reactive epitopes because it stimulates TC lines restimulated in vitro with M. tuberculosis. These epitopes include both the known ones, such as the 65-kDa protein epitopes, and those which are as yet undefined. Some authors have described the 65-kDa protein of M. leprae as "cell wall associated" (7), while others have proposed a periplasmic location and have shown the release of it into culture supernatants of M. bovis (3). In our study, 65-kDa protein-reactive TC clones were strongly stimulated by the M. leprae cell wall PPC, comparable with that seen by whole M. leprae. This indicates that at least the part(s) of the 65-kDa protein that contain the epitopes for these TC clones are still present in PPC.
Acknowledgments. We would like to thank Dr. P. J. Brennan for providing the peptidoglycan-protein complex (PPC), Dr. R.J. W. Rees for M. leprae antigen, and Anncke Janson and Dienne Elferink-Bontrop for technical advice and assistance. This study was supported in part by the Immunology of Leprosy (IMMLEP) component of the UNDP/World Bank/ WHO Special Programme for Research and Training in Tropical Diseases and The Netherlands Leprosy Relief Association (NSL).
REFERENCES
1. Bloom, B. R. and Godal, T. Selective primary' health care: strategies for control of disease in the developing world. V. Leprosy. Rev. Infect. Dis. 5(1983)765-780.
2. Carbone, F. R., Fox, B. S., Schwartz, R. H. and Paterson, Y. The use of hydrophobic, alpha-helix-defined peptides in delineating the T cell determinant for pigeon cytochrome c. J. Immunol. 138(1987)1838-1844.
3. de bruyn, J., bosmans, R., TuRNEER, M., WeCKX, M., Nyabenda, J., van Vooren, J.-P., Falmange, P., Wiker, H. G. and Harboe, M. Purification, partial characterization and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect. Immun. 55(1987)245-252.
4. Draper, P. The walls of Mycobacterium lepraemurium: chemistry' and ultrastructure. J. Gen. Microbiol. 69(1971)313-324.
5. Draper, P. Cell walls of Mycobacterium leprae. Int. J. Lepr. 44(1976)95-98.
6. Enoers, H. D., Abe, M., Bloom, B. R., Mehra, V., Britton, W., Buchanan, T. M., Khanolkar, S. K., Young, D. B., Closs, O., Gillis, T. P., Harboe, M., Ivanyi, J., Kolk, A. H. J. and Shep-hard, C. C. Results of a WHO sponsored workshop on monoclonal antibodies to Mycobacterium leprae. Infect. Immun. 48(1985)603-605.
7. Gillis, T. P., Miller, R. A., Young, D. B., Khanolkar, S. R. and Buchanan, T. M. Im-munocharactcrization of a protein associated with Mycobacterium leprae cellwall. Infect. Immun. 49(1985)371-377.
8. Hunter, S. W., McNeil, M., Modlin, R. L., Mehra, V., Bloom, B. R. and Brennan, P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J. Immunol. 142(1989)2864-2872.
9. Mehra, V., Bloom, B. R., Torigian, V. K., Mandich, D., Reichel, M., Young, S. M. M., Salgame, P., Convit, J., Hunter, S. W., McNeil, M., Brennan, P. J., Rea, T. H. and Modlin, R. L. Characterization of Mycobacterium leprae cell wall-associated proteins with the use of T lymphocyte clones. J. Immunol. 142(1989)2873-2878.
10. Melancon-Kaplan, J., Hunter, S. W., McNeil, M., Stewart, C, Modlin, R. L., Rea, T. H., Convit, J., Salgame, P., Mehra, V., Bloom, B. R. and Brennan, P. J. Immunological significance of Mycobacterium leprae cell walls. Proc. Natl. Acad. Sci. U.S.A. 85(1988)1917-1921.
11. Misaki, A., Yukawa, S., Tsuchiya, K. and Ya-masaki, T. Studies on cell walls of Mycobacteria. I. Chemical and biological properties of the cell walls and the mucopeptide of BCG. J. Biochem. (Tokyo) 59(1966)388-396.
12. Modlin, R. L., Kato, H., Mehra, V., Nelson, E. E., Fan, X.-D., Rea, T. H., Pattengale, P. K. and Bloom, B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. Nature 322(1986)459-461.
13. Mustafa, A. S., Gill, H. K., Nerland, A., Britton, W. J., Mehra, V., Bloom, B. R., Young, R. A. and Godal, T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature 319(1986)63-66.
14. Myrvang, B., Godal, T., Ridley, D. S., Fro-land, S. S. and Song, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathologic spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
15. Ottenhoff, T. H. M., Klatser, P. R., Ivanyi, J., Elferink, D. G., de Witt, M. Y. L. and de Vries, R. R. P. Mycobacterium leprae specific protein antigens defined by human helper T cells. Nature 319(1986)66-68.
16. Ridley, D. S. and Jopling, W. L. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
17. Rook, G. A. W. and Stewart-Tull, D. E. S. The dissociation of adjuvant properties of mycobacterial components from mitogenicity, and from the ability to induce the release of mediators from macrophages. Immunology 31(1976)389-396.
18. Spouge, J. L., Guy, H. R., Cornette, J. L., Mar-galit, H., Cease, K., Berzofsky, J. A. and DeLisi, C.Strong conformational propensities enhance T cell antigenicity. J. Immunol. 138(1987)204-212.
19. Stewart-Tull, D. E. S. The immunological activities of bacterial peptidoglycans. Ann. Rev. Microbiol. 34(1980)311-340.
20. Thole, J. E. R., van Schooten, W. C. A., Keulen, W. J., Hermans, P. W. M., Janson, A. A. M., de Vries, R. R. P., Kolk, A. H. J. and Embden, J. D.A. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect. Immun. 56(1988)1633-1640.
21. Young, R. A., Mehra, V., Sweetser, D., Buchanan, T. M., Clark-Curtiss, J., Davis, R. W. and Bloom. B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316(1985)450-152.
1. M.D.; Department of Immunohematology and Bloodbank, University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands.
2. M.Sc; Department of Immunohematology and Bloodbank, University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands.
3. Ph.D., M.D., Department of Immunohematology and Bloodbank, University Hospital, P.O. Box 9600, 2300 RC Leiden, The Netherlands.
Reprint requests to Dr. de Vries.
Received for publication on 26 May 1989.
Accepted for publication on 20 June 1989.