- Volume 57 , Number 4
- Page: 801–9
Skin test studies on close contacts of leprosy patients in India
ABSTRACT
Skin-test studies with a series of tuberculins have been carried out in close contacts of multibacillary (MB) leprosy patients around three leprosy centers in India, and in casual contacts of the disease around two centers. The results show that the rate of acquisition of leprosin A positivity is associated with age and the closeness of contact with MB leprosy. At the age of 15 years, the differences between the two types of contact were highly significant (p < 0.00001). Many responses to leprosin A are directed toward the group iv, species-specific , antigens of the leprosy bacillus, and the significance of positivity is discussed in relation to protective immunity f rom leprosy. The differences f rom Iran show that positivity to leprosin A is not solely the effect of the degree of contact with the disease, but must also have a genetic or environmental element, the latter being favored.The results f rom Miraj show that the high levels of tuberculin, scrofulin, and vaccin positivity seen in Fathimanagar. and to a lesser extent in Karigiri, are not a consequence of contact with leprosy. BCG vaccination made little difference to the leprosin A positivity of close contacts of leprosy patients, although it significantly enhanced positivity among casual contacts around Miraj (p < 0.002). BCG vaccination significantly increased tuberculin positivity in Miraj and Karigiri, and in those under 11 years of age in Fathimanagar. It made no difference to the already high level of positivity found in older persons around Fathimanagar. Gender was found to influence skin-test responses in close contacts tested around Fathimanagar who were BCG vaccinated, but made no difference among the nonvaccinated.
When used with care, leprosin A is a valuable tool for the study of the effects of contact with leprosy, and illustrates the high infectivity of the organism, yet comparatively low incidence of disease.
RÉSUMÉ
Des études d'épreuves cutanées ont été menées avec une série de tuberculines d'une part chez des contacts étroits de malades atteints de lèpre multibacillaire (MB), autour de trois centres de lutte contre la lèpre en Inde, et d'autre part chez des contacts occasionnels autour de deux de ces centres. Les résultats indiquent que les taux de virage positif de la léprosine A sont associés à l'âge ainsi qu'au le degré de contact avec la lèpre multibacillaire. A partir de l'âge de 15 ans, les taux notés chez l'un et l'autre de ces types de contacts présentaient des différences hautement significatives (p < 0,00001). Un grand nombre des réponses à la léprosine A étaient dirigées contre les antigènes du bacille de la lèpre appartenant au groupe IV, qui est spécifique pour l'espèce. La signification de ces résultats positifs est discutée en rapport avec l'immunité protectrice contre la lèpre. Les différences enregistrées en Iran ont révélé que le caractère positif à la léprosine A ne résultait pas seulement du degré de contact avec la maladie, mais qu'il faisait également intervenir des éléments génétiques ou de milieu, ces derniers étant les plus importants.Les résultats obtenus à Miraj montrent que les taux élevés de résultats positifs à la tuberculine, à la scro-fuline, et à la vaccine relevés à Fathimanagar, et également mais dans une moindre mesure à Karigiri, ne sont pas la conséquence d'un contact avec la lèpre. La vaccination par le BCG ne fait guère de différence quant au caractère positif de la réaction à la léprosine A chez des contacts étroits de malades de la lèpre; néanmoins, autour de Miraj, cette vaccination avait renforcé de manière significative la positivité chez des contacts occasionnels (p < 0,002). La vaccination par le BCG a entraîné une augmentation significative des taux positifs à la tuberculine à Miraj et à Karigiri, et également chez les sujets agés de moins de 11 ans à Fathimanagar. Par contre, cette vaccination n'a pas eu d'effet lorsque les taux positifs étaient déjà fort élevés, comme c'est le cas chez les personnes agées autour de Fathimanagar. On a observé que le sexe influençait les réponses à l'épreuve cutanée chez les personnes en contact étroit autour de Fathimanagar, lorsque celles-ci étaient vaccinées. La vaccination ne faisait aucune différence chez les non-vaccines.
Lorsqu'elle est utilisée avec circonspection, la léprosine A est un outil utile pour étudier les effets du contact avec la lèpre. Elle illustre le caractère infectieux qui possède ce microorganisme en dépit d'une incidence relativement faible de la maladie.
RESUMEN
Se midió la reactividad en piel en contactos íntimos de pacientes con lepra multibacilar (MU) hacia una serie de tuberculinas en 3 centros leprológicos en la India y en contactos casuales de la enfermedad en dos centros. Los resultados mostraron que la frecuencia de adquisición de positividad a la leprosina A, está asociada con la edad y con la cercanía del contacto con la lepra MU. A la edad de 15 años, las diferencias entre los dos tipos de contactos fueron altamente significativas (p < 0.00001). Muchas respuestas a la leprosina A estuvieron dirigidas hacia el grupo iv de antígenos especic-especific os de los bacilos de la lepra y la significancia de la positividad se discute en relación a la inmunidad protectora contra la lepra. Las diferencias encontradas con respecto a Iran, muestran que la positividad hacia la leprosina A no es únicamente el efecto del grado de contacto con la enfermedad sino que también puede existir un factor genético o ambiental, favoreciéndose este último.Los resultados de Miraj muestran que los altos niveles de positividad a la tuberculina, a la escrofulina y a la vaccinia observados en Fathimanagar, y en menor grado en Karigiri, no son consecuencia del contacto con la lepra. La vacunación con BCG prácticamente no modificó la positividad a la leprosina A de los contactos cercanos de los pacientes con lepra, aunque si incrementó significativamente la positividad de los contactos casuales en Miraj (p < 0.002). La vacunación con BCG aumentó significativamente la positividad a la tuberculina en Miraj y en Karigiri, y en aquellos contactos menores de 11 años en Fathimanagar. La vacunación no modificó el ya alto nivel de positividad en personas de mayor edad en Fathimanagar. El sexo influyó en la reactividad dérmica de los contactos cercanos del área de Fathimanagar que fueron vacunados con BCG, pero no modificó la respuesta en los no vacunados.
Cuando se usa con cuidado, la leprosina A es una herramienta útil en el estudio de los efectos del contacto con la lepra, e ilustra la alta infectividad del organismo en contraste con la relativamente baja incidencia de la enfermedad.
Although most new cases of leprosy occur in individuals not known to be in close contact with leprosy patients, the chances of developing the disease are greatest in the households of patients with multibacillary (MB) leprosy. The incidence of new cases decreases as the degree of contact with known patients diminishes. In order to determine some of the effects of contact with patients with MB leprosy we have skin tested members of the patients' families and children attending schools in leprosy-endemic areas of India. Particular attention has been paid to the results obtained with the soluble reagents leprosin A and tuberculin. The study has been carried out around Karigiri, and Fathimanagar in Tamil Nadu and around Miraj in Maharashtra, India.
MATERIALS AND METHODS
Population studied. The study based in Karigiri was carried out in 1976, the study based on Miraj was performed in 1985, and the study based on Fathimanagar 20 kilometers from Tiruchirappalli was carried out in 1987-1989.
The people studied were divided into those living in households including a patient with MB leprosy and called "close contacts," and those without known contact with leprosy patients but living in a leprosy-endemic area. These latter will be called "casual contacts." All of the patients with MB disease were cither receiving antilepro-sy therapy or had finished their treatment after becoming bactcriologically negative. Both Karigiri and Fathimanagar are in districts with leprosy prevalences above 10 per 1000; Miraj is in a district with a leprosy prevalence of about 5 per 1000.
The age of those studied ranged from less than 1 year to 70 years, the majority of them being under 25 years old.
Reagents. The reagents used were new tuberculins prepared from Mycobacterium tuberculosis (tuberculin), M. scrofulaceum (scrofulin), and M. vaccae (vaccin) from organisms grown on Sauton's medium (12), and leprosin A (batch CD 73) prepared from M. leprae obtained from experimentally infected armadillos (1). The batches of reagents used were the same in Miraj and Fathimanagar, but earlier batches were used in Karigiri. Except for children under 3 years of age and a few older children, who were tested with only two reagents, everyone was tested with four reagents, two on each forearm. In the Karigiri study only three of the test reagents (scrofulin was not used) were used in combinations with various other new tuberculins (results not reported). In the later studies only the four reagents described above were used. Tuberculin (0.2 μg) was given in the left upper position; leprosin A (1 μg) was given at least 10 cm away in the left lower position. Scrofulin (0.2 μg)was given in the right upper position, and vaccin (2 μg) in the right lower position. In every case 0.1 ml doses of each reagent were injected intradermally at least 10 cm apart, two tests per forearm. The results were read as two diameters of the area of induration 72 hr after injection, and a mean diameter of 2 mm or more was considered positive (2,7,16,18).
BCG scars. BCG scars were sought on every individual at the time of skin testing; recorded as "BCG positive" if a scar was present, and "BCG negative" if it was not.
Handling of results. The results were analyzed according to whether individuals were, or were not, known to be household contacts of patients with MB forms of leprosy, even if their treatment had been completed and they were bacteriologically negative. They were also analyzed according to whether the individual had a BCG scar, and where there were sufficient numbers, according to age and sex. Statistical analysis was by Fisher's exact test or by Student's t test.
To find out how many of those tested with all four reagents were reacting to group i (13), common mycobacterial, antigens (i.e., antigens that all species of mycobacteria share), categorization of responder types was carried out (7). In this system, category 1 individuals respond to all four reagents,"most of them reacting to group i antigens. Category 2 persons fail to respond to all four reagents; in most cases this is thought to be due to failure (suppression ?) of mediator release in skin-test sites. Category 3 people respond to some reagents and not to others, through reaction to the group iv, species-specific , antigens of the mycobacteria they have met. The observed proportion of category 1 persons was corrected for those positive for all reagents by chance, and 20% were found to truly respond to group i antigens. An estimate of the percentage of positive responses that are to group iv, species-specific , antigens in a given reagent was then made among the category 3 responders.
RESULTS
The results for numbers tested, mean ages, percentages positive to each reagent and mean positive reaction sizes are shown for the three study areas in Tables 1, 2 and 3 and in Figures 1 and 2.
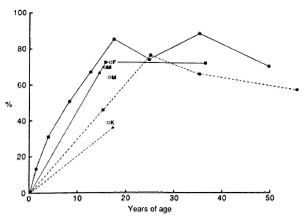
Fig. 1. Acquisition of leprosin A positivity with age among BCG nonvaccinated close contacts ( ) and casual contacts (-----) of leprosy patients around Karigiri (
) and casual contacts (-----) of leprosy patients around Karigiri ( ), Miraj (
), Miraj ( ), and Fathimanagar (
), and Fathimanagar ( ). Single point results for BCG recipient close contacts (
). Single point results for BCG recipient close contacts ( ) and casual contacts (
) and casual contacts ( ) are marked with the initial of the centers where the data were obtained. Although the points are linked to make interpretation easier, it should be noted that different groups were tested at the different ages.
) are marked with the initial of the centers where the data were obtained. Although the points are linked to make interpretation easier, it should be noted that different groups were tested at the different ages.
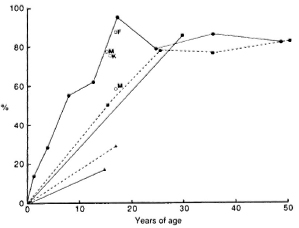
Fig. 2. Acquisition of tuberculin positivity with age among BCG nonvaccinated close contacts ( ) and casual contacts (-----) of leprosy patients around Karigiri (
) and casual contacts (-----) of leprosy patients around Karigiri ( ), Miraj (
), Miraj ( ), and Fathimanagar (
), and Fathimanagar ( ). Single point results for BCG recipient close contacts (
). Single point results for BCG recipient close contacts ( ) and casual contacts (
) and casual contacts ( ) are marked with the initial of the centers where the data were obtained. Although the points are linked to make interpretation easier, it should be noted that different groups were tested at the different ages.
) are marked with the initial of the centers where the data were obtained. Although the points are linked to make interpretation easier, it should be noted that different groups were tested at the different ages.
By the age of 15 years, 70% of close contact children without BCG scars were positive responders to leprosin A in all three centers. In Fathimanagar (Table 3, Fig. 1), the only center where we have sufficient data, leprosin A positivity in those without BCG scars reached a maximum of 89% at the age of 35 years and declined to 70% by the age of 50 (p < 0.02). The rate of acquisition of positivity in these children was 11.5% in the first year, 6.5% per year over the next 3 years, and then it steadily decreased. In Ka-rigiri and Miraj, where we studied individuals not known to be close contacts of leprosy, only 46% and 36%, respectively, were positive to leprosin A by the age of 15 years. In Karigiri where sufficient casual contact individuals were studied, a peak in leprosin A positivity of 76% occurred at the age of 25 years, and declined to 57% by the age of 55 (Fig. 1). The difference between leprosin A positivity for close contacts and casual contacts around the age of 15 years was highly significant (p < 0.00001).
BCG significantly enhanced leprosin A positivity among the casual contacts studied in Miraj (from 11/31 to 115/176; p < 0.002). However, its effect was disappointing among the close contacts at Fathimanagar (from 42/49 to 29/40) and Miraj (from 8/12 to 16/23) and the casual contacts in Karigiri (from 104/226 to 9/23).
In Fathimanagar, among the close contacts of leprosy patients tuberculin positivity steadily rose from 15% at the age of 15 months to a plateau around 85% between the ages of 17 to 50 years (Fig. 2). The single point of tuberculin data for close contacts from Karigiri indicated that the pattern may be similar there (p = 0.4); in Miraj, tuberculin positivity was only 17% by the age of 15 years (p < 0.0001). After BCG vaccination tuberculin results for close contacts in Miraj and Fathimanagar were the same. Among casual contacts of leprosy patients, tuberculin positivity was higher in Karigiri than in Miraj in both those with and without BCG scars (p < 0.03).
Scrofulin and vaccin positivity in close contacts followed a pattern similar to leprosin A positivity in Fathimanagar. Scrofulin positivity in Miraj and vaccin positivity in both Karigiri and Miraj were considerably less than in Fathimanagar.
The sizes of the reactions to all of the reagents increased with age up to 20 years and then remained about the same over the next 20 years, perhaps falling a little thereafter.
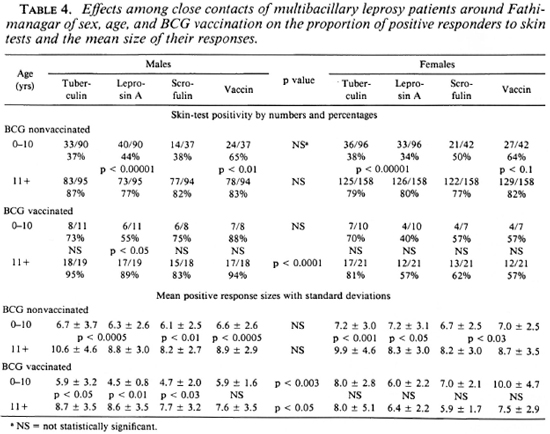
The influence of sex and age on the effects of BCG vaccination was investigated at Fathimanagar (4), and the results are shown in Table 4. Sex made no significant difference at all to those without BCG scars, but ageing significantly increased positivity (p < 0.01 to 0.00001 for different reagents) and mean positive reaction sizes (p < 0.05 to 0.0005 for different reagents). In those with BCG scars, sex made no significant difference to positivity to any reagent in children under 11 years old, but above this age women were less positive to each reagent than were the men (p < 0.0001). For both sexes of BCG recipients increasing age was associated with greater positivity to most reagents, but this only reached significance for leprosin A among males (p < 0.05). Sex differences were found in the sizes of the reactions in BCG recipients. Girls under 11 years of age produced larger responses than did boys (p < 0.003); above 11 years men produced larger responses than did women (p < 0.05). There were no differences in response sizes between the two age groups of females, but in males responses were larger in the older age group with differences reaching significance for all of the reagents except vaccin (p < 0.05 to 0.01). BCG significantly enhanced positivity to tuberculin in both sexes in the younger age group, but the effect was not significant in the older age group.
The proportion of category 1 persons responding to group i, common mycobacterial, antigens was calculated (data not shown) to be no more than 20% in any group. Thus, much of the responsiveness to leprosin A in both close contacts and casual contacts was to antigens specific to the leprosy bacillus (3,15).
DISCUSSION
In all three centers close contact with MB leprosy was associated with the rapid acquisition of skin-test positivity to leprosin A, reaching 70% positivity by the age of 15 years. In Karigiri and Miraj where casual contacts were studied, positivity to leprosin A was significantly less than in the close contacts (p < 0.00001), reaching only about 40% by the age of 15 years. These observations strongly link leprosin A positivity to contact with leprosy bacilli in the surroundings of MB patients (10,11). The presence of a BCG scar made no significant difference to leprosin A positivity among close contacts in Miraj or Fathimanagar, but it was effective in increasing leprosin A positivity among the casual contacts in Miraj (p < 0.002). The small number of casual contacts with BCG scars tested in Karigiri indicated that it was less successful there, suggesting that the enhancement found in Miraj was unlikely to be due solely to responsiveness to group i, common mycobacterial, antigens.
The decline in leprosin A positivity with age (Fig. 1) in close contacts of both sexes at Fathimanagar and in casual contacts at Karigiri follow a curve of similar shape. It would be interesting to know whether this decline, which may be due to the effects of many years' contact with large numbers of environmental mycobacteria, is associated with the increase in prevalence of leprosy found in older persons. A similar decline is seen in scrofulin (p < 0.05) and vaccin (p < 0.03) positivity in the close contacts at Fathimanagar (Table 3).
As might be expected, responsiveness to tuberculin is different at the different centers (Fig. 2), presumably dependent on the local frequency of tuberculosis. The degree of contact with leprosy had no effect on tuberculin positivity in cither Karigiri or Miraj, although the proportion positive to tuberculin was significantly greater in Karigiri than in Miraj (p < 0.01). BCG vaccination boosted tuberculin positivity in the close contacts in Miraj and in the casual contacts in both Karigiri and Miraj, but was effective only in those less than 11 years old among the close contacts in Fathimanagar, where tuberculin positivity was very high in those without scars.
Categorization of skin-test responders showed little difference between Fathimanagar and Miraj after correction for the numbers expected to fall into categories 1 and 2 by chance (3,7). Thus, most of the differences in skin-test responsiveness are due to reactions to group iv, species-specific , antigens (13). This lends further support to the conclusion that contact with M. leprae itself is the inducement for leprosin A positivity in our study.
Our findings are in striking contrast to those from Azerbaijan in Iran (2,16,18). Prior to BCG vaccination less than 6% of Iranian children (mean age 4.6 years), who were in very close contact with leprosy patients, and less than 8% of children (mean age 9.9 years), who were casual contacts of the disease, were positive to leprosin A. In both groups of Iranian children BCG vaccination was associated with marked increases in leprosin A positivity, but it must be noted that the period of follow up may have been much longer in Iran than in this study. The batch of leprosin A (CD 73) used in Iran was the same as that used in Fathimanagar, and in a proportion of cases the person carrying out the tests was the same.
The differences between the Indian and the Iranian results must lie in race or environment, since a difference in the organism causing the disease in the two countries seems unlikely. In the face of the known differences in environmental mycobacterial content between the areas studied in the two countries, this would seem to hold the most probable explanation. In Iranian Azerbaijan there is only moderate contact with environmental mycobacteria, illustrated by the small percentages of children without BCG scars responding to scrofulin and vaccin (2,18). In South India contact with such organisms is very frequent (9,14). Regular contact with such organisms may prime the individual to recognize contact with M. leprae by becoming positive to leprosin A, exerting the same mode of action as that expected from BCG vaccination (8,17).
The most important aspect of our study depends on whether there is an association between the level of positivity to leprosin A and protective immunity from leprosy in a population. Analogous responses to tuberculin do seem to be associated with protection from tuberculosis. Thus in Britain, where BCG vaccination confers 80% protection from tuberculosis (5,6), 85% of BCG recipients are tuberculin positive 10 years after vaccination; whereas only 23% of the nonvaccinated population of the same age are tuberculin positive. However, among the BCG recipients in Britain, individuals who had no palpable skin-test response to tuberculin were just as well protected from tuberculosis as were tuberculin-positive individuals (5,6).
Recent studies, in which skin-test sites were biopsied 48 hr after injection, have shown that similar numbers of cells of the same types may be present in some reactions without detectable induration as are seen in normal positive responses. The induration appears to be a function of mediator release rather than the volume of infiltrating cells. If it is the cellular infiltrate that is important for protective immunity, there is no reason why every protected individual should give an indurated response to tuberculin, and yet tuberculin positivity still remains a valuable marker of resistance to infection in a population. By analogy, the same may be true for leprosin A, since the greatest proportion of positive responders was found among healthy close contacts of the infectious patients. Cellular infiltration at the site of injection of leprosin A undoubtedly indicates an immune reaction to antigens of the leprosy bacillus, but it is not yet certain whether those particular antigens in the reagent to which the response is directed are presented by living bacilli, or whether the cellular reaction to such antigens is appropriate to the containment or destruction of bacilli.
In conclusion, we report significant differences between close and casual contacts of multibacillary leprosy patients in their responses to quadruple skin testing, and wc find leprosin A to be a valuable reagent for their study. Although more direct evidence is needed, it docs seem that leprosin A positivity may be a useful marker of immune protection from developing disease among the healthy population. Nonetheless, caution is needed in the interpretation of a positive response in an individual.
Acknowledgments. We should like to thank all those who allowed us to skin test them for their forebearance, and all those leprosy workers who assisted our studies. We are very grateful to LEPRA which has unfailingly given financial support, without which the work could not have been done.
REFERENCES
1. Draper, P. and Rees, R. J. W. Proposed system for preparing purified suspensions of M. leprae from tissues of infected armadillos. Report of the second IMMLEP task force meeting, December 1975, Protocol number 2/75. Lepr. Rev. 47(1976)320-323.
2. Ghazi Saidi, K., Stanford, J. L., Stanford, C. A., Dowlati, Y., Farshchi, Y., Rook, G. A. W. and Rees, R. J. W. Vaccination and skin test studies on children living in villages with differing endemicity for leprosy and tuberculosis. Int. J. Lepr. 57(1989)45-53.
3. Lockwood, D. N. J., McManus, I. C, Stanford, J. L., Thomas, A. and Abeyagunawardana, D. V. P. Three types of response to mycobacterial antigens. Eur. J. Respir. Dis. 71(1987)348-355.
4. MeIntyre, G., Belsey, E. and Stanford, J. L. Taxonomic differences between Mycobacterium avium and M ycobactcrium intraccllulare elucidated in man by skin tests with three new tuberculins. Eur. J. Respir. Dis. 69(1986)146-152.
5. Medical Research Council of Great Britain, Tuberculosis Vaccines Clinical Trials Committee. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescents; second report. Br. Med. J. 2(1959)379-396.
6. Medical Research Council of Great Britain, Tuberculosis Vaccines Clinical Trials Committee. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescents; third report. Br. Med. J. 1(1963)973-978.
7. Nye, P. M., Price, J. E., Revankar, C. R., Rook, G. A. W. and Stanford, J. L. The demonstration of two types of suppressor mechanism in leprosy patients and their contacts by quadruple skin-testing with mycobacterial reagent mixtures. Lepr. Rev. 54(1983)9-18.
8. Rook, G. A., Bahr, G. M. and Stanford, J. L. The effect of two distinct forms of cell-mediated response to mycobacteria on the protective efficacy of BCG. Tubercle 62(1981)63-68.
9. Shield, M.J. The importance of immunologically effective contact with environmental mycobacteria. In: Biology of the Mycobacteria, Volume 2; Immunological and Environmental Aspects. Rat-ledge, C. and Stanford, J. L., eds. London: Academic Press, 1984, pp. 343-415.
10. Shield, M. J. and Stanford, J. L. The epidemiological evaluation, in Burma, of the skin test reagent LRA6; a cell-free extract from armadillo-derived Mycobacterium leprae. Part 2: Close contacts and non-contacts of bacilliferous leprosy patients. Int. J. Lepr. 50(1982)446-454.
11. Shield, M. J., Stanford, J., L., Garbajosa, G., Draper, P. and Rees, R. J. W. The epidemiological evaluation, in Burma, of the skin test reagent LRA6; a cell-free extract from armadillo-derived Mycobacterium leprae. Part 1: Leprosy patients. Int. J. Lepr. 50(1982)436-445.
12. Shield, M. J., Stanford, J. L., Paul, R. C. and Carswell, N. W. Multiple skin testing of tuberculosis patients with a range of new tuberculins, and a comparison with leprosy and Mycobacterium ulcerans infection. J. Hyg. 78(1977)331-348.
13. Stanford, J. L. and Grange, J. M. The meaning and structure of species as applied to mycobacteria. Tubercle 55(1974)143-152.
14. Stanford, J. L. and Rook, G. A. W. Environmental mycobacteria and immunisation with BCG. In: Medical Microbiology, Volume 2: Immunisation Against Bacterial Disease. Easmon, C. S. F. and Jeljaszcwicz, J., eds. London: Academic Press, 1983, pp. 43-70.
15. Stanford, J. L., Rook, G. A. W., Convit, J., Godal, T., Kronvall, G., Rees, R. J. W. and Walsh, G. P. Preliminary taxonomic studies on the leprosy bacillus. Br. J. Exp. Pathol. 56(1975)579-585.
16. Stanford, J. L., Rook, G. A. W., Samuel, N., Madlener, F., Khamenei, A. A., Nemati, T., Modabber, F. and Rees, R. J. W. Preliminary immunological studies in search of correlates of protective immunity carried out on some Iranian leprosy patients and their families. Lepr. Rev. 51(1980)303-314.
17. Stanford, J. L., Shield, M. J. and Rook, G. A. W. How environmental mycobacteria may predetermine the protective efficacy of BCG. Tubercle 62(1981)55-62.
18. Stanford, J. L., Stanford, C. A., Ghazi Saidi, K., Dowlati, Y., Weiss, Sister F., Farshchi, Y., Madlener, F. and Rees, R. J. W. Vaccination and skin test studies on the children of leprosy patients. Int. J. Lepr. 57(1989)38-44.
19. Swanson Beck, J., Morley, S. M., Gibbs, J. H., Potts, R. C, Ilias, M. I., Kardjito, T., Grange, J. M., Stanford, J. L. and Brown, R. A. The cellular responses of tuberculosis and leprosy patients and of healthy controls in skin tests to "new tuberculin" and leprosin A. Clin. Exp. Immunol. 64(1986)484-494.
1. M.B.B.S.; former students at Middlesex Hospital Medical School, Royal Free Hospital Medical School, St. Bartholomew's Hospital Medical College, respectively.
2. M.B.B.S.; former students at Middlesex Hospital Medical School, Royal Free Hospital Medical School, St. Bartholomew's Hospital Medical College, respectively.
3. M.B.B.S., former students at Middlesex Hospital Medical School, Royal Free Hospital Medical School, St. Bartholomew's Hospital Medical College, respectively.
4. S.R.N., School of Pathology, University College and Middlesex School of Medicine, Ridinghouse Street, London W1P 7LD, U.K.
5. M.D., School of Pathology, University College and Middlesex School of Medicine, Ridinghouse Street, London W1P 7LD, U.K.
6. M.D., Ph.D., Schieffelin Leprosy Research and Training Centre. Karigiri 632106, Tamil Nadu, South India.
7. M.D., D.D., Schieffelin Leprosy Research and Training Centre. Karigiri 632106, Tamil Nadu, South India.
8. M.B.B.S., M.P.H., Richardson Leprosy Hospital, Miraj 416410 Maharashtra, India.
9. M.B.B.S., The Holy Family Hansenorium, Fathimanagar, Tiruchi-rappalli 621316, Tamil Nadu, South India.
10. M.B.B.S., The Holy Family Hansenorium, Fathimanagar, Tiruchi-rappalli 621316, Tamil Nadu, South India.
11. M.D., The Sacred Heart Leprosy Centre, Kumbako-nam, Sakottai 612401, Tamil Nadu, South India.
12. M.B.B.S., F.R.C.P., F.R.C.Path., National Institute for Medical Research. Mill Hill, London, U.K.
Present address for Dr. Debanbu: Gremaltes Referral Hospital and Leprosy Centre, Shenoy Nagar, Madras 600030, India.
Reprint requests to Dr. J. L. Stanford.
Received for publication on 22 March 1989.
Accepted for publication in revised form on 5 June 1989.