- Volume 57 , Number 4
- Page: 810–6
Implementation of chemoprophylaxis of leprosy in the Southern Marquesas with a single dose of 25 mg per kg rifampin
ABSTRACT
Between 1967 and 1987 in the Southern Marquesas, a remote archipelago in French Polynesia, the detection rate of leprosy was 48.9 per 100,000 when it was 8.6 per 100,000 for French Polynesia as a whole. In 1988, a program of chemoprophylaxis of leprosy with a single dose of 25 mg/kg rifampin was implemented, and 2751 persons (98.7% of the population) were treated in the Southern Marquesas. In addition, 678 South Marquesans and 2466 members of their families, living in the Northern Marquesas and in the Society Archipelago, received the same chemoprophylaxis. Among 2676 persons studied in the Southern Marquesas (97.4% of the treated population), 130 had elevated IgM anti-phenolic glycolipid-I antibodies by ELISA without any evidence of leprosy. The onset of a skin lesion of borderline leprosy in a boy 3 months after chemoprophylaxis raises the question of the nature of such a skin lesion and, indirectly, of the effectiveness of the chemoprophylaxis.RÉSUMÉ
Dans les Marquises du Sud, un archipel éloigné en Polynésie française, le taux de détection de la lèpre de 1967 à 1987 s'est élevé à 48,9 pour 100.000, alors qu'il était de 8,6 pour 100.000 dans l'ensemble de la Polynésie. En 1988, un programme de chimioprophylaxie de la lèpre a été entrepris, comprenant l'administration d'une dose unique de 25 mg/kg de rifampicine. Aux Marquises du Sud, 2751 personnes (98,7% de la population) ont été traitées de cette manière. En outre, 678 habitants des îles Marquises du Sud, et 2466 membres de leur famille vivant dans les Marquises du Nord et dans l'archipel de la Société, ont reçu la môme chimioprophylaxie. Parmi les 2676 personnes étudiées dans les îles Marquises du Sud (97,4% de la population traitée), 130 présentaient des taux élevés d'anticorps IgM anti-phéno-glycolipidique-I, révélés par une méthode ELISA, alors qu'il ne présentaient aucune signe clinique de lèpre. Par ailleurs, l'apparition d'une lésion cutanée de lèpre dimorphe chez un garçon de trois mois soulève la question de la nature d'une lésion cutanée de ce type et met en cause de manière indirecte l'efficacité de cette chimioprophylaxie.RESUMEN
Se encontró que la incidencia de lepra, entre 1967 y 1987, en las Marquesas del Sur, un remoto archipiélago en la Polinesia Francesa, fue de 48.9 por 10,000, mientras que ésta fue de 8.6 por 100,000 en toda la Polinesia Francesa. En 1988 se implemento un programa de qui-mioprofilaxis contra la lepra consistente en una sola dosis de 25 mg/kg de rifampina y se trataron 2751 personas (98.7% de la población) en las Marquesas del Sur. Además, 678 Sur-Marquesinos y 2466 de sus familiares residentes en las Marquesas del Norte y en el Archipiélago Sociedad, recibieron la misma quimio-profilaxis. Se encontró que de las 2676 personas estudiadas en las Marquesas del Sur (97.4% de la población tratada), 130 tuvieron niveles elevados de anticuerpos IgM anti-glicolipido fenólico-I (por ELISA) sin ninguna evidencia de lepra. La aparición de una lesión de tipo intermedio en la piel de un niño de 3 meses, después de la quimioprofilaxis, plantea la necesidad de establecer la naturaleza de esa lesión y, de manera indirecta, la necesidad de establecer la efectividad de la quimioprofilaxis.To reach the main goals of leprosy control, it is necessary to interrupt the transmission of Mycobacterium leprae in the community (22). For this purpose, the current strategy is based on effective chemotherapy of early detected cases. Chemoprophylaxis might also be considered. Studies conducted in India have demonstrated that the 12-month chemoprophylaxis of child contacts with dapsone given twice weekly had an efficacy rate of 42% to 55%. The conclusion of these studies was that mass chemoprophylaxis of a contact population was not likely to bring a significant reduction in the incidence of leprosy. However, if chemoprophylaxis was given to the entire population in an endemic area it might contribute to leprosy control (11-13). Rifampin being a drug much more active than dapsone against M. leprae (8,10,16-19), one may wonder whether chemoprophylaxis of the entire population with a single 25 mg/kg dose of rifampin would not be bacteriolog-ically more effective and operationally more feasible than a long course of dapsone alone. Such a strategy might be best in remote islands with a high incidence of leprosy and as a supplement of the multidrug therapy of all known cases of leprosy. The aim of this paper is to report how such a chemoprophylaxis, never tested elsewhere, was implemented in the Southern Marquesas, remote archipelagoes of French Polynesia highly endemic for leprosy (2) with a population of limited size, and the results of the serological tests that were performed simultaneously.
MATERIALS AND METHODS
Material Located some 1500 km north from Tahiti, the main island of French Polynesia, the Marquesas Archipelago comprises six inhabited islands, the three southernmost of which are the Southern Marquesas. From the data of demographic censuses performed in 1977 and 1983 (9), their population was estimated by December 1987 to be 2796.
Although a leprosarium was built in the Southern Marquesas in 1918, in order to gather the patients from the Northern and Southern Marquesas, leprosy control has not relied upon special facilities in the Southern Marquesas since 1935 when the leprosarium closed (15,20). The Marlardé Institut in Papeete, Tahiti, is in charge of the central register and the control of leprosy for the entire French Polynesia, including the Southern Marquesas. Its action is completed by local health authorities (two medical doctors, a dozen nurses, and a small hospital in the Southern Marquesas). Multidrug therapy (MDT) of 6-months duration for paucibacillary (PB) leprosy and of 24-months duration for multibacillary (MB) leprosy has been applied since 1982, with high individual effectiveness (no relapses have been observed in treated patients) but without any significant effect on the new case detection rate. As shown in Table 1, between 1967 and 1987, 255 new cases of leprosy were detected in French Polynesia, of whom 29 were born in the Southern Marquesas. The average annual detection rate in the Southern Marquesas is 48.9 per 100,000, nearly two new patients per year, significantly higher (p < 0.001) than the 8.6 overall average detection rate for all of French Polynesia.
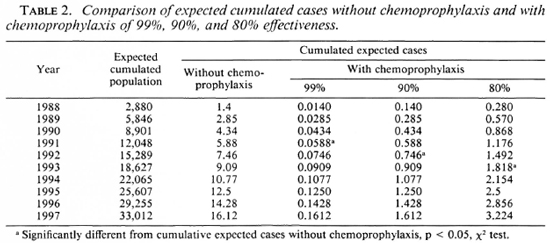
Methods. To measure what could be expected from a chemoprophylaxis program in the Southern Marquesas, an epidemio-metric projection was established based on cases detected in the past 21 years. The Figure shows the reduction in the number of expected cases if the chemoprophylaxis was of 80%, 90%, and 99% effectiveness. According to that epidcmiometric projection, a significant decrease in the leprosy detection rate in the Southern Marquesas could be expected in as little as four years (Table 2).
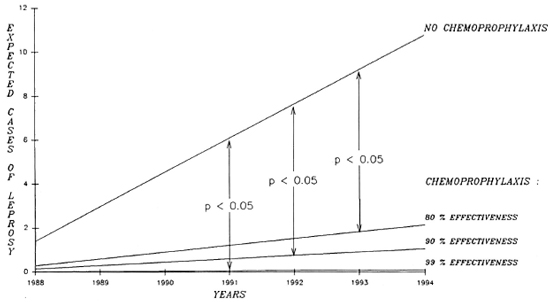
The Figure. Cumulated expected cases of leprosy without chemoprophylaxis and with chemoprophylaxis of 99%, 90%, or 80% effectiveness.
During 1987, all villages in all of the valleys of the three inhabited Southern Marquesas islands were visited by the team in charge of the chemoprophylaxis program in order to explain to the population and the local authorities the rationale of the chemoprophylaxis. All known leprosy patients had a clinical and bacteriological examination, and household contacts of these patients were examined. Also, an updating of the 1983 demographic census of the population born in the Southern Marquesas and living either in the Southern Marquesas or elsewhere in French Polynesia was performed. For the three Southern Marquesas islands the number of inhabitants was calculated to be 2695; a list of these 2695 persons was edited by island, valley and village, providing also for each person the family name, given names, sex and date of birth.
Using this list, two teams consisting of a medical doctor, a nurse, and a health worker of the leprosy control unit, supplemented with a South Marquesan primary health worker, started implementation of the chemoprophylaxis on 18 January 1988 and finished by 20 February 1988. Each person received a supervised dose of 25 mg/kg rifampin (capsules for adults and syrup for children), and the body weight of each treated person was registered.
To monitor the effect of the chemoprophylaxis in addition to the identification of new cases of leprosy, finger-prick blood samples were collected from all subjects receiving the chemoprophylaxis to determine anti-phenolic glycolipid-I (anti-PGL-I) IgM antibodies by the ELISA method using the natural trisaccharide 3-p-hydroxy-phenyl-propionate-bovine serum albumin (NTP) as antigen (3,5). In the assay, a cut-off level of 0.200 optical density (OD) was chosen because of results of previous studies performed in French Polynesia (4). In addition to the chemoprophylaxis for the Southern Marquesas inhabitants, chemoprophylaxis was also given to South Marquesans living in the Northern Marquesas and Society Archipelago (including Tahiti) between February and July of 1988. No finger-prick blood samples were collected from this latter population.
RESULTS
During a visit organized in the fall of 1987, before implementation of chemoprophylaxis, five patients who had inactive leprosy after long courses of dapsone monotherapy and who were skin-smear negative were put on WHO MDT (21). For all of the other 24 known patients who were already on MDT or had been on MDT, no treatment changes were needed.
The entire chemoprophylaxis program was implemented in the Southern Marquesas in 4 weeks. Although the list of inhabitants had been updated in advance, the total number of inhabitants was 2786 not 2695. Of the 2786 inhabitants, 2751 (98.7%) attended the program and were given rifampin chemoprophylaxis. Among them, 2676 (97.4%) accepted the blood sample collection. In the Northern Marquesas and Society Archipelago, 3144 persons were also given chemoprophylaxis because they were born in the Southern Marquesas (678) or were members of a family from the Southern Marquesas (2466).
During the chcmoprophylaxis program, cutaneous reactions were observed in the treated population. These reactions consisted of flushing of the face, the neck and the shoulders with itching. They appeared 30 min after the intake of rifampin and disappeared in about 1 hr spontaneously or after administration of an antihistaminic drug. On the main island of the Southern Marquesas, among a treated population of 1662, 49 of these reactions were observed (2.9%). Of these, 5 occurred in 835 males (0.6%) and 44 in 827 females (5.3%).
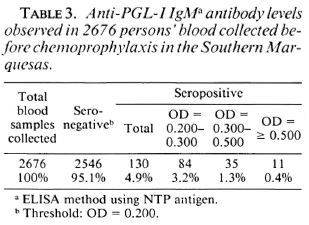
The results of the anti-PGL-I IgM assays performed at the Marlarde Institut in Papeete are given in Table 3. Among the 2676 blood samples, 2546 (95.1%) were considered negative; 130 (4.9%) were positive. Of the latter, 84 (3.2%) had an OD ranging from 0.200 to 0.300, 35 (1.3%) had an OD ranging from 0.300 to 0.500, and 1 1 (0.4%) had an OD > 0.500. Clinical and bacteriological examinations as well as measurement of the PGL-I antigen levels (6) in sera (collected by venipuncture after chcmoprophylaxis) were performed for the 11 persons with the highest antibody levels. All examinations were negative.
Finally, by June 1988, a skin lesion was observed in an 11-year-old boy, the son of a South Marquesan living in Tahiti, who had been given chemoprophylaxis in February 1988. The lesion was a large, infiltrated oval patch, warm and of coppery aspect, located on the back part of the right arm. The search for acid-fast bacilli (AFB) was positive in the lesion (4+) and in the nasal mucosa (4+) and negative in the ear-lobes; no solid-staining bacilli were found. A biopsy was taken for histopathological examination and for inoculation into the mouse foot pad. The conclusions of the histological examination were: borderline (BB-BL) leprosy, presence of AFB (2+). The ba-cillary load in the biopsy, measured at the time of the inoculation into the mouse foot pad, was 2 x 104 M. leprae per mg; the morphological index was 0%. At the time the lesion was discovered, another blood sample was collected for determination of anti-PGL-I IgM and PGL-I antigen levels; the results of the two tests were negative.
Moreover, because he was a household contact of a leprosy patient detected in 1980, this boy had had a yearly medical examination since 1985, including blood collection for determination of anti-PGL-I IgM levels. The results of the tests performed in 1985, 1986, and 1987 were all negative.
DISCUSSION
The results reported demonstrate that, when epidemiological conditions are suitable (small population and high detection rate of leprosy) as in the Southern Marquesas, a chemoprophylaxis program is possible and acceptable. Of the 2786 Southern Marquesas inhabitants, 2751 have received chemoprophylaxis -98.7% coverage. In addition, the program permitted the examination of known patients and their family members and a change in treatment when needed. Nevertheless, such a chemoprophylaxis program requires considerable resources for its implementation. For a population of 2786 inhabitants in these remote islands, the total cost of the program including the demographic census, the visit to the villages prior to chemoprophylaxis and the chemoprophlaxis itself, was over US$50,000.
The side effects in the population treated with a dose as high as 25 mg/kg rifampin were acceptable and did not jeopardize the program. They were not different in nature and overall frequency from those described by Girling and Hitze (7). The only surprising point is the different frequency observed in males (0.6%) and in females (5.3%), which could be due to the fact that the concentration of rifampin is nearly two times higher in the sera of females than in the sera of males (14).
The results of the immunological assays (Table 3) involve 96.1% of the population and can be considered as a picture of the immunological status of the Southern Marquesas inhabitants before chcmoprophylax-is. Of the 130 seropositive persons, 11 showed an anti-PGL-1 IgM level at OD 0.500 or more, that is the lower level observed in MB patients in French Polynesia (4). In these 1 1 persons no signs of leprosy were found, and the PGL-I antigen assays were negative. This could mean, as it has been reported in a similar study (1), that anti-PGL-I IgM determination is of limited value for detection of new cases of leprosy. On the other hand, seropositive persons could be infected persons at an early stage of infection. This hypothesis will be tested in the coming years because the 46 seropositive persons with an OD > 0.300 and the 138 seronegative randomized persons will be tested again at 6-month intervals over the next 4 years.
The onset of a skin lesion with a high bacterial index in an 1 1-year-old boy 2 months after chemoprophylaxis raises the question of the nature of such a skin lesion and, indirectly, of the effectiveness of the prescribed chemoprophylaxis. Actually, a slightly discolored patch was observed in July 1987 on the right deltoid area of the boy 9 months before chemoprophylaxis. No sensory loss was detected at the site of the lesion or in its bordering area. No AFB were found in the smears taken from the lesion, the nasal mucosa, and both earlobes. Because the boy was a household contact of a leprosy patient, he was requested to come for a new examination 3 months later. At that time, in October 1987, the skin lesion had vanished and consequently the boy was given the same chemoprophylaxis given to all other South Marquesans in February 1988 without further skin examination. Since the skin lesion that occurred in June 1988 was located at the very same site as the preceding patch, the likely conclusion is that the boy was a missed case of leprosy and not a failure of chemoprophylaxis. The fact that the results of both anti-PGL-I IgM and PGL-I antigen determination were negative, even at the moment the skin lesion was observed, is not really surprising. Regarding the negativity of anti-PGL-I IgM level, it is known that this test can be negative in about 20% of the patients with borderline leprosy (5). Regarding the negativity of the PGL-I antigen level, it must be pointed out that, for our patient, the test was performed with sera collected after the intake of rifampin and a rapid decrease of PGL-I antigen has been reported in leprosy patients treated with rifampin (6).
Acknowledgments. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases and from the following ILEP organizations: Fondations Raoul Follereau (France), Leprosy Trust Board (New Zealand), and Oeuvres Hospitalières Françaises de l'Ordre de Malte (France). We are indebted to the staff of the Public Health Service in the Marquesas Islands for their assistance in the field, especially Dr. J.-C. Genette, P. Richez and D. Hebral; Mrs. V. Matuaiti, D. Schlegel, and M.-L. Tetahiotupa; and Mr. F. Vaatete and D. Ah Lo.
REFERENCES
1. Baumgart, K., Britton, W., Basten, A. and Bagshawe, A. Use of phenolic glycolipid 1 for serodiagnosis of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81(1987)1030-1032.
2. Cartel, J-L., Boutin, J-P., Plichart, R., Roux, J. and Grosset, J-H. La lèpre dans les archipels de Polynésie Française de 1967 à 1987. Bull. Soc. Pathol. Exot. Filiales 81(1988)819-826.
3. Chanteau, S., Cartel, J-L., Roux, J., Plichart, R. and Bach, M. A. Comparison of synthetic antigens for detection of antibodies to phenolic glycolipid 1 in patients with leprosy and their household contacts. J. Infect. Dis. 4(1988)770-776.
4. Chanteau, S., Cartel, J-L., Roux, J., Plichart, R. and Bach, M-A. PGL1 antigen and antibody detection in the control of leprosy in French Polynesia. Acta Leprol. (Geneve) (1988) (in press).
5. Cho, S. N., Fujiwara, T., Hunter, J. W., Rea, T. H., Gelber, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3, 6-di-o-meth-yl-β-d-glucopyranosyl epitope for th serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
6. Cho, S. N., Hunter, S. W., Gelber, R. H., Rea, T. H. and Brennan, P. J. Quantitation of the phenolic glycolipid of M. leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153(1986)560-569.
7. Girling, D.J. and Hitze, K. L. Adverse reactions to rifampicin. Bull. WHO 57(1979)45-49.
8. Grosset, J-H. Recent developments in the field of multidrug therapy and future research in chemotherapy of leprosy. Lepr. Rev. 57 Suppl. 3( 1986)223-234.
9. Institut Territorial de la Statistique. Recensements généraux de la population en 1967, 1971, 1977 et 1983. I.T.Stat. BP 395. Papeete. Tahiti.
10. Levy, L., Shepard, C. C. and Fasal. P. The bactericidal effect of rifampicin on M. leprae in man: a) single doses of 600, 900 and 1200 mg ; and b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
11. Noordeen, S. K. Chemoprophylaxis in leprosy. Lepr. India 40(1968)115-119.
12. Noordeen, S. K. Long-term effects of chemoprophylaxis among contacts of lepromatous cases. Lepr. India 49(1977)504-509.
13. Noordeen, S. K. and Neelan, P. N. Extended studies on chemoprophylaxis against leprosy. Indian J. Med. Res. 67(1978)515-527.
14. Porven, G. and Canetti, G. Les taux de rifampicine dans le sérum de l'homme. Rev. Tuberc. Pneumol. 32(1968)707-716.
15. Sasportas, L. La lèpre dans les établissements français de l'Océanic. Imprimerie du Gouvernement, Papeete, 1924. (Archives de l'Institut Ma-lardé).
16. Shepard, C.C. A survey of the drugs with activity against M. leprae in mice. Int. J. Lepr. 39(1971)340-346.
17. Shepard. C. C. Recent developments in the chemotherapy and chemoprophylaxis of leprosy. Leprologia (Argent.) 19(1974)230-236.
18. Shepard, C. C., Levy, L. and Fasal, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1124.
19. Subcommittee on Clinical Trials of the Chemotherapy of Leprosy (THELEP) Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. THELEP controlled trials in leproma-tous leprosy. Lepr. Rev. 54(1983)167-173.
20. Valleteau De Moulliac. Etude Sur la lèpre à Tahiti. Imprimerie du Gouvernement. Papeete, 1912. (Archives de l'Institut Malardé).
21. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneve: World Health Organization, 1982. Tech Rep. Sen 675.
22. WHO Study Group. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
1. M.D.; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
2. Ph.D.; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
3. M.D.; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
4. Computer Scientist; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
5. M.D., Professor; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
6. M.D., Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
7. M.D., South Pacific Commission, P.O. Box D5, Noumea, New Caledonia.
8. M. D., Professor, Faculté de Médecine Pitié Salpêtrière, 91 Blvd. de l'Hôpital, 75613 Paris, France.
Received for publication on 27 December 1988.
Accepted for publication in revised form on 19 May 1989.