- Volume 57 , Number 4
- Page: 867–70
Serum ferritin in lepra reactions
To the Editor:
During an inflammatory process in the body, there is a fall of serum iron levels and a release of various acute phase reactants (4). Ferritin behaves as an acute phase reactant, and it is a measure of the body's iron stores (5). Hyperferritinemia has been observed in inflammatory conditions, such as Hodgin's disease, carcinomas, osteomyelitis, tuberculosis (1), and AIDS (5). Serum iron and ferritin levels were comparable in lepromatous patients and control subjects (9), while their levels were remarkably decreased in lepromatous patients co-infected with pulmonary tuberculosis (12). Here we report the serum ferritin levels in type 1 and type 2 lepra reactions. These complications are often encountered even after the introduction of multidrug therapy (MDT) in India (15).
We have measured serum ferritin levels by radioimmunoassay (9) in leprosy patients at Singhbhum Navjivan Niketan, Bihar; Gandi Memorial Leprosy Foundation, Wardha; and the Dermatology Department of Maulana Azad Medical College. New Delhi. India. Age- and sex-matched controls from the same areas were also included for comparison. Twenty patients (male and female) with nonreactional lepromatous leprosy (10) with a mean age of 40.5 years (Table 1) and another 29 patients of both sexes with type 1 and type 2 lepra reactions (7) (Table 2) were studied. The duration of illness in all of these patients varied from 3 months to 25 years. In the type 1 reaction cases, there were 1 upgrading BL, 1 BT and 2 BB downgrading cases*, the remaining 10 cases were either unstable or uncertain. The clinical details of patients in lepra reaction are given in Table 2. The control group consisted of 20 normal male and female subjects, with a mean age of 36.5 years. All patients received MDT (15). The patients in lepra reaction were managed with steroids, clofazimine, chloroquin and/or aspirin (8). Paired serum samples were collected from the reactional patients, one at onset prior to the antireaction therapy and the other at clinical remission of the reaction.
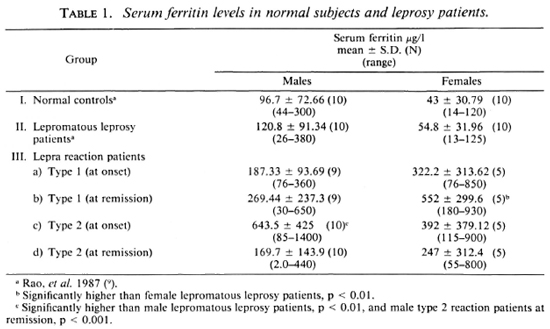

The serum ferritin levels in the different groups of subjects are given in Table 1. The differences in mean serum ferritin levels between males and females in both the normal controls (Group I) and lepromatous patients (Group II) were not significant (p > 0.1). However, in the males the mean serum ferritin levels were twice that found in the females. None of the control subjects or nonreactional lepromatous patients had a deficiency of serum ferritin (< 12 μg/l).
At the onset of type 1 reaction, only 4 of 14 (29%) cases showed elevation of serum ferritin levels; at the start of type 2 reaction, 11 of 15 (73%) cases demonstrated elevated levels. Values > 320 μg/l in the males and > 125 in the females, i.e., the upper limits of nonreactional lepromatous patients, were taken as raised (Table 1). At the onset, the rise of the mean serum ferritin level was more in type 2 than in type 1 lepra reaction. However, this difference is not significant. At the clinical remission of type 1 reaction, the serum ferritin levels declined to the normal range in 1 BL case and 1 BB case while, curiously, in 5 other cases showing no rise at onset, the levels were raised (Table 2). At the clinical subsidence of type 2 reaction, only four patients still had elevated serum ferritin levels. The fall of the mean serum ferritin level at remission of the type 2 reaction was remarkable (Table 1), its level being significantly less than that at onset (p < 0.001).
Reactions in leprosy are consequent to the disregulation in the immunological homeostasis. Differentiation of upgrading and downgrading reactions is possible histologically, provided a previous biopsy report is available (2), which is not always the case. In our previous studies, we had tried to differentiate type 1 and 2 lepra reactions by studying the levels of serum Cortisol (15) and serum-β-2-microglobulin (11) and also by the solubilizing capacity of preformed immune complexes (an indicator of complement activation) by the sera of lepromatous patients (3), at the clinical onset and remission of lepra reactions. Some overlapping of these parameters prompted us to search further for means of delineating type 1 and 2 lepra reactions by laboratory tests.
We found that a larger number of cases (73%) showed a sudden and spectacular elevation of serum ferritin levels at the onset of the type 2 reaction and demonstrated an accelerated fall at remission in most cases. On the other hand, in type 1 reaction only 29% of cases showed elevation at the onset and, surprisingly, at remission 50% of the cases showed elevated levels. The rise in the serum ferritin levels during reaction is perhaps due to damage to reticuloendothelial cells, leading to the release of stored ferritin into the circulation. This indicates that the possibility of acute systemic involvement and tissue destruction may be more common in type 2 lepra reaction, which is quickly extinguished by antireactional drugs. To the contrary, type 1 reaction might be a localized immunologic struggle, which perhaps gradually intensifies. However, very high levels of serum ferritin in some cases of type 1 reaction (Table 2) possibly point to immunological instability and a gradual worsening or downward shift of cellular immunity in these patients. The fall of serum ferritin at the remission of type 2 reaction suggests a settling of the immunoinflam-matory processes. It may be further noted that only 4 out of 15 patients with type 2 reaction had minimally elevated serum ferritin levels. These patients had perhaps milder Gell and Coombs type III hypersensitivity, with marginal systemic involvement. To the contrary, hyperferritincmia at the onset of type 1 reaction indicates a Gell and Coombs type IV hypersensitivity reaction at the site of the lesions. The increased level of serum ferritin in 7 out of 14 cases of type 1 reaction at clinical remission cither indicates a downward shift of the cellular immunity or, alternatively, a relapse. Leprologists are aware of the difficulty in distinguishing relapse in paucibacillary patients from late reversal reaction (14).
Patients near the lepromatous end of the leprosy spectrum may also undergo overlapping type 1 and 2 reactions. Thus, the complexity of the clinical picture may make the distinction between these two types of reaction difficult (7). We had two such patients grouped in type 2 reaction in our scries. Admittedly, these cases could not be differentiated by serum ferritin estimations.
So far as we know, until now there is no laboratory method except histology to gauge the severity of lepra reactions. Although the elevation of the serum ferritin level in lepra reactions is an indicator of nonspecific inflammatory processes, our preliminary data warrant a further in-depth study on the rise and fall of this acute phase reactant during lepra reaction.
- Kunal Saha, M.Sc, M.B.B.S.,Ph.D.
K. N. Rao, Ph.D.
Avind Kashyap, M.Sc.
Immunology Department
Vallabhbhai Patel Chest Institute
Delhi 110007, India
- V. N. Sehgal, M.D., F.N.A.Sc.
S. K. Agrawal, M.D.
Department of Dermatology and Medicine
Maulana Azad Medical College
New Delhi 110002, India
Acknowledgment. The authors are grateful to the Indian Council of Medical Research, New Delhi, for financial assistance.
REFERENCES
1. Birgegard, G., Hallgren, R., Killander, A. and Stomberg, A. Serum ferritin during infection. Scand. J. Haematol. 21(1978)333-340.
2. Bryceson, A. and Pfaltzgraff, R. E. Leprosy. 2nd ed. Edinburgh: Churchill Livingstone, 1979, pp. 65-71.
3. Chakrabarty, A. K., Kashyap, A., Sehgal, V. N. and Saha, K. Solubilization of preformed immune complexes in sera of patients with type 1 and 2 lepra reactions. Int. J. Lepr. 56(1988)559-565.
4. Connolly, K. M., Stacher, V. J., LaBrie, T. and Fluno, C. Modulation of acute phase response and in vitro measurement of interleukin-1 activity following administration of dexamethasone to adjuvant arthritic rats. Immunopharmacology 15(1988)133-142.
5. Gupta, S., Imam. A. and Licorish, K. Serum ferritin in acquired immunodeficiency syndrome. J. Clin. Lab. Immunol. 20(1986)11-14.
6. Hollbrand, A. V. and Pettit, J. E. Iron deficiency and other hypoehronic anemias. In: Essential Haematology. Oxford: Blackwell Scientific Publications, 1980, pp. 28-41.
7. Jopling, W. H. Handbook of Leprosy. 2nd ed. London: William Heinemann Medical Books, 1978, pp. 66-74.
8. Manson-Bahr, P. E. C. and Apted, F. I. C. Manson's Tropical Diseases. 18th ed. London: English Language Book Society. 1982, pp. 298-322.
9. Rao, K. N., Saha, K. and Chakrabarty, A. K. Undernutrition and lepromatous leprosy. III. Micronutrients and their transport proteins. Hum. Nutr. Clin. Nutr. 41(1987)127-134.
10. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
11. Saha, K., Bhatnagar, A., Sharma, V. K. and Chakrabarty, A. K. Enzyme immunoassay of serum beta-2-microglobulin levels in various histological forms of leprosy with special reference to its elevation in type I and type II lepra reactions. J. Clin. Microbiol. 21(1985)658-661.
12. Saha, K. and Rao. K. N. Undernutrition in lepromatous leprosy. Part V: Severe nutritional deficit in lepromatous patients co-infected with pulmonary tuberculosis. Eur. J. Clin. Nutr. 43(1989)117-128.
13. Saha, K., Rao, K. N., Sehgal, V. N., Gadi, S., Jain, V. K. and Chakrabarty, A. K. Radioimmunoassay of serum Cortisol levels in leprosy patients with reference to type I and II reaction. Lepr. Rev. 56(1985)117-125.
14. Van Brakel, W., Kist, P., Noble, S. and O'Toole, L. Relapse after multidrug therapy for leprosy: a preliminary report of 22 cases in West Nepal. Lepr. Rev. 60(1989)45-50.
15. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.