- Volume 57 , Number 4
- Page: 870–2
lnterleukin-2 receptors in the sera of leprosy patients
To the Editor:
A variety of immunological changes occur in borderline tuberculoid leprosy progressing toward the lepromatous pole (4). A particular deficit in the number and functions of T cells has been seen in step-ladder fashion from the tuberculoid to the lepromatous end. On the other hand, B-cell activity is generally noted to be elevated as we shift from the tuberculoid to the leproma-tous pole (12). B cells are activated by antigens of Mycobacterium leprae with accompanying hypergammaglobulinemia associated with the appearance of autoantibodies also to a variety of tissue components (14). It has been suggested that a portion of the autoantibodies bind to the T-helper-cell population (3). Apart from interleukin-1 (IL-1), interlcukin-2 (IL-2) plays an important role in the generation of T- and B-cell immune reactions (8). As a result of antigenic stimulation of T lymphocytes, IL-2 is produced and receptors for lymphokines are also expressed (2). IL-2 is also necessary for the sustained long-term growth of T cells and for the generation of cytotoxic T-cell responses (16). Rubin, et al. (9) demonstrated the accumulation of soluble IL-2 receptors (IL-2R) in the culture supernatant when human T lymphocytes were stimulated in vitro with mitogen, antigen or anti-T3 antibody. The same group of workers (10) reported elevated levels of circulating IL-2R in patients with T-cell leu-kemias. The quantification of IL-2R in the sera of leprosy patients could therefore be a useful indicator of T-cell-mediated responses, and hence was carried out in the present study.
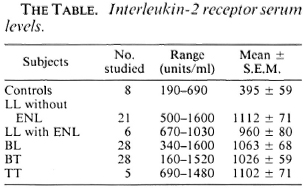
Patients. Eighty-eight patients registered in the Leprosy Clinic of Nehru Hospital attached to the Postgraduate Institute of Medical Education and Research, Chandigarh, India, were studied. They included 21 patients with lepromatous leprosy (LL), 6 with LL undergoing erythema nodosum lepro-sum (ENL), 28 with borderline lepromatous (BL), 28 with borderline tuberculoid (BT), and 5 with tuberculoid (TT) leprosy. Five ml samples of venous blood were drawn from each patient. The sera were separated and stored at -70ºC until the assay was carried out. Samples similarly drawn and stored were obtained from eight healthy controls.
IL-2R assay. The quantification of IL-2R levels in the serum samples was done in duplicate using the cell-free Interleukin-2 Receptor Test Kit (T Cell Sciences, Inc., Cambridge, U.K.).
The range of IL-2R in healthy individuals was 190-690 units/ml with an average of 395 units/ml (S.E.M. 59.7). A significant elevation (p < 0.001) of IL-2R was seen in all of the leprosy patients, regardless of the clinical type of the disease (The Table).
Discussion. Normal resting T and B lymphocytes display very few IL-2 receptors on their surface. Antigenic stimulation results in their division and expression on the cell's plasma membrane. A form of the IL-2R protein which is 10 kDa smaller than the usual 65 kDa membrane-bound IL-2 is also released into the surrounding tissue fluid, the significance of which is not yet known but is also detectable by the system used.
Elevated levels of soluble IL-2R have been shown in a number of pathological conditions (10), in certain autoimmune diseases (1), and in viral infections, the classical example being the acquired immunodeficiency syndrome (11). However, few studies are available regarding the levels of soluble IL-2R in leprosy. Tung, et al. (13) reported significantly higher levels of IL-2R in patients of the multibacillary type while paucibacil-lary patients had significantly lower levels compared to the controls, possibly suggesting that the potential for development of delayed-type hypersensitivity responses to M. leprae antigens as such is insufficient to elicit more IL-2 receptors in the serum. Modlin, et al. (7) have found equal numbers of IL-2R-bearing cells in tuberculoid and lepromatous lesions, but Longley, et al. (5)found decreased numbers of IL-2R cells in lepromatous lesions.
The responsiveness of B cells to IL-2 can also be induced by appropriate activation. Tsudo, et al. (15) reported that human B cells stimulated with Staphylococcus aureus (Cowan strain I) for 3 days proliferated in response to affinity-purified IL-2. Activated B cells were shown to express IL-2 on their surface and anti-IL-2R blocked the proliferative response to IL-2. Other workers have confirmed this for both murine (17) and human (6) activated B cells.
In the present study, elevated levels of soluble IL-2R were seen in all types of lep-rosy. The increase observed in the ÎL-2 receptors in the sera of leprosy patients could be due to several reasons: a) antibodies might be generated against IL-2R which bind to the specific receptors and the complexes are extruded into the circulation by the activated cells; b) IL-2 produced by M. leprae-activated T cells might be structurally defective and unable to attach to the 1L-2R and thereby enters the circulation; c) IL-2R are produced by cells other than OKT4+, especially 13 cells which are known to increase in lepromatous leprosy Studies conducted on these lines would probably give some insight into the immune reaction against M. leprae.
- Nirmal Kumar Ganguly, M.D., F.A.M.S.
Chetana Vaishnavi, Ph.D.
Department of Experimental Medicine
- Surrinder Kaur, M.D., F.A.M.S.
Nalini Agnihotri, M.Sc.
Bhushan Kumar, M.D., M.N.A.M.S.
Department of Dermatology
Postgraduate Institute of Medical Education and Research
Chandigarh 160012, India
REFERENCES
1. Burmester, G. R., Jahn, B., Gramatzki, M. and Kalden, J. R. Analysis of rheumatoid synovial T cells expressing the receptor for interleukin-2 (IL-2) and/or the la antigen: different phenotypic expression as compared to in vitro activated T cells. Abstract in Scand. J. Rheumatol. 12 Suppl. 49(1983)31.
2. Cantrell. D. A. and Smith, K. A. The interleukin-2 T cell system: a new cell growth model. Science 224(1984)1312-1316.
3. Dorsett, B., Cronin, W., Chuma, V. and Ioa-chim, H. L. Anti-lymphocyte antibodies in patients with the acquired immune deficiency syndrome. Am. J. Med. 78(1985)621-626.
4. Godal, T., Myklestad, B., Samuel, D. R. and Myrvang. B. Characterisation of the cellular immune defect in lepromatous leprosy; a specific lack of circulating M. leprae reactive lymphocytes. Clin. Exp. Immunol. 9(1971)821-831.
5. Longley, B. J., Haregewoin, A., de Beaumount, W., Smith, K. A. and Godal, T. Lepromin stimulates interleukin-2 production and interleukin-2 receptor expression in situ in lepromatous leprosy patients. Lepr. Rev. 57 Suppl. 1(1986)189-198.
6. Minoari, M. C. Gerosa. F., Carra, G., Ac-colla, R. S., Moretta, A., Zuhler, R. H., VVald-mann, T. A. and Moretta, L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated t cells. Nature 312(1984)641-643.
7. Modlin, R. L., Hofman. F. M., Horowitz, D. A., I Iusmann, L. A., Gillis, S., Taylor, C. R. and Rea, T. H. In situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin 2 and its receptor. J. Immunol. 132(1984)3085-3090.
8. Robb, R. J. Interleukin-2: the molecule and its function. Immunol. Today 5(1984)203-209.
9. Rubin. L. A., Kurman, C. C, Fritz, M. E., bid-dison, W. F., Boutin, B., Yarchoan, R. and Nelson, D. Soluble interleukin-2 receptors are released from activated human lymphoid cells in vitro. J. Immunol. 135(1985)3172-3177.
10. Rubin, L. A., Kurman, C. C, Fritz, M. E., Yarchoan, R. and Nelson, D. L. Identification and characterisation of a released form of interleukin-2 receptor. In: Leukocytes and Host Defense. Oppenhein, J. J. and Jacobs, D. M., eds. New York: Allen R. Liss, Inc., 1986, pp. 95-102.
11. Sethi, K. K. and Naher, H. Elevated titers of cell-free interleukin-2 receptor in serum and cerebrospinal fluid specimens of patients with acquired immunodeficiency syndrome. Immunol. Lett. 13(1986)179-184.
12. Sharma, S., Ganguly, N. K., Kumar, B., Kaur, S. and Chakravarti, R. N. T and B lymphocytes and histogenesis in leprosy. Lepr. India 51(1979)194-202.
13. Tung, K. S. K., Umland, E., Matzner, P., Nelson, K., Schauf, V., Rubin, L., Wagner, D., Scollard, D., Vithayasai, P., Vithayasai, V., Worobec, S., Smith, T. and Suriyanand, V. Soluble serum interleukin 2 receptor levels in leprosy patients. Clin. Exp. Immunol. 69(1987)10-15.
14. Turk, J. L. and Bryceson, A. D. M. Immunological phenomena in leprosy and related diseases. Adv. Immunol. 13(1971)209-266.
15. Tsudo, M., Uchiyama, T. and Uchino, H. Expression of Tac antigen on activated normal B cells. J. Exp. Med. 160(1984)612-617.
16. Wagner, H., Hardt. C, Heeg, K., Rollinghoff, M. and Pfizenmaier, K. T-cell-dcrived helper factor allows in vivo induction of cytotoxic T cells in nu/nu mice. Nature 284(1980)278-280.
17. Zubler, R. H., Lowenthal, J. W., Eserd, E., Hashimoto, N., Devos, R. and MacDonald, H. R. Activated B cells express receptors for and proliferate response to pulse interleukin-2. J. Exp. Med. 160(1984)1170.