- Volume 57 , Number 4
- Page: 879–82
Glomerular immunoglobulin deposition in the absence of tissue damage in murine leprosy
To the Editor:
A study was undertaken to gain insight into the immunopathology of the kidneys in murine leprosy, a very neglected subject. Despite the fact that murine leprosy is a visceral, disseminated disease, the kidneys are usually spared. In advanced murine leprosy the kidneys appear, at most, pale and enlarged, and when lesions are found they are limited to the outer aspect of the capsule. In rare cases, bacilli and leprous lesions are found in the glomeruli, Bowmann's capsule, and ducti controiti of the renal parenchyma (1). In our study, a group of 10 albino, adult, NIH mice were inoculated intraperitoneally with 108 Mycobacterium lepraemurium (MLM) Hawaiian strain harvested from previously infected mice. Six months after inoculation, the animals were sacrificed and examined for pathological external and internal changes. Their kidneys were excised and divided in half: one half was collected in 10% Formalin in 0.01 M phosphate-0.15 M NaCl (PBS), pH 7.4; the other was embedded in OCT compound (Miles, Elkhart, Illinois, U.S.A.), frozen on dry ice, and stored at -70ºC until used.
Histological examination of the Formalin-fixed kidneys was performed following standard paraffin sectioning. Two-micron-thick sections were stained with the he-matoxylin-cosin, Masson's trichrome, Jones' silver reticulum, and periodic-acid Schiff techniques.
For the immunohistochemical studies, 5-μm-thick frozen sections were fixed in chilled acetone for 10 min, air dried, rehydrated, washed in PBS, and stained with one of the following: a) fluoresceinated (FITC)-rabbit antibody to mouse immunoglobulins, b) FITC-rabbit and mouse antibody to MLM, c) FITC-goat antibody to mouse C3b, d) horseradish peroxidase (PO)-labeled rabbit antibody to mouse IgG, e) PO-goat antibody to mouse IgM, or f) PO-goat antibody to mouse C3c. The antibodies to mouse immunoglobulins were from Cap-pel Laboratories, West Chester, Pennsylvania, U.S.A.; anti-mouse C3b and C3c were from Nordic Immunological Laboratories, Tilburg, The Netherlands; anti-MLM antibodies were prepared in our laboratory. All of the above antibodies were previously titrated on appropriate substrates and used at dilutions of 1:20 and 1:40. The sections stained for 30-60 min with the FITC-an-tibodies were counterstained with 0.125% Evan's blue in water for 30 min (2), washed in PBS, mounted in glycerol containing 2% propyl gállate (3), and examined under incident ultraviolet light. The sections to be stained with the PO-labeled antibodies were treated with periodic acid for 45 sec to eliminate intrinsic PO activity (4), incubated for 30 min with normal rabbit serum, then with the corresponding PO-antibody for 30 min (with PBS washings between steps), and the presence of PO-labeled-reacted antibodies detected by incubation of the slides for 20 min in a solution containing 0.125 M phosphate buffer, pH 6.0 (24 ml), o-dianisidinc (3.175 mg),and 1% H2O2 (30 μl). After rinsing in water, the sections were counter-stained with Harris' hematoxylin with a 5-sec acid decoloration (1% HC1 in 70% ethanol), and a 30-scc alkaline bluing (2 drops concentrated ammonium hydroxide in 50 ml water), washed in water, dried, immersed in xylol for 1 min to clear the tissue, and mounted in synthetic resin for examination. Acid-fast bacilli (AFB) were looked for by staining the sections with the hot phenol-fuchsin reagent as previously described (5).
Histological examination by light microscopy showed no important alterations in the kidney tissue of MLM-infected animals. Some focal thickening of the glomerular basal membrane, some increase of the mesangial matrix, and some vascular congestion were the only noticeable anomalies found in some animals. No tissue damage was observed despite the fact that in the histochemical examination, 7 out of the 10 MLM-infected animals showed marked glomerular deposition of both IgG and IgM, with a predominance of IgG (Figs. 1 and 2). Complement (C3b and C3c) deposition was not detected in any of the examined kidneys, and the presence of mycobacterial antigen was erratic in spite of the fact that the FITC-labeled anti-MLM antibody used in the study clearly revealed the presence of mycobacterial antigen in the lepromatoid granulomas of the liver and other affected structures (Fig. 3). As reported previously (6), pretreatment of tissue sections with a solution containing lysozyme and lipase (8 mg each in 40 ml 0.06 M phosphate buffer, pH 6.8) was necessary to obtain optimal reactivity with the FITC-antibodies to MLM. AFB were only rarely found in the glomeruli or in other structures of the cortical renal parenchyma in one animal, but larger collections of AFB were seen as small lepromas in the outer aspect of the kidney capsule and, more frequently, as lepromatoid granulomas adjacent to the medullary blood vessels at the hiliar region.
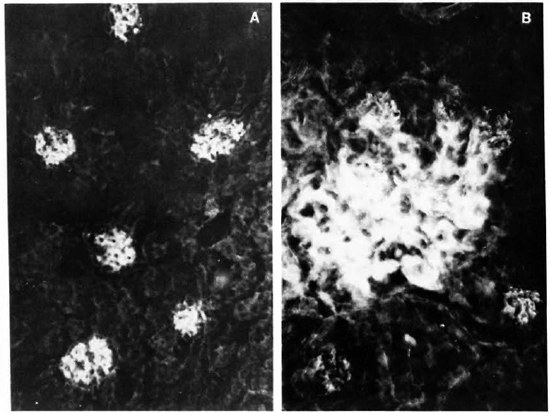
Fig. 1. A. Renal section from mouse infected with MLM, stained with a fluoresceinated rabbit antibody to mouse immunoglobulins. Six glomeruli appear-clearly fluorescent (x 500).
B. Higher magnification of a glomerulus in A, showing a mesangial pattern of immunoglobulin deposition (immersion oil x 125).
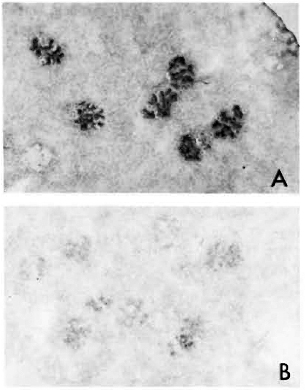
Fig. 2. A. Kidney section from MLM-infected mouse, stained with a peroxidase-labeled rabbit antibody to mouse IgG. Similar result but weaker staining was observed with peroxidase-labeled goat antibody to mouse IgM.
B. Kidney section from a matched healthy mouse treated with peroxidase-anti-mouse IgG antibody ( x 125).
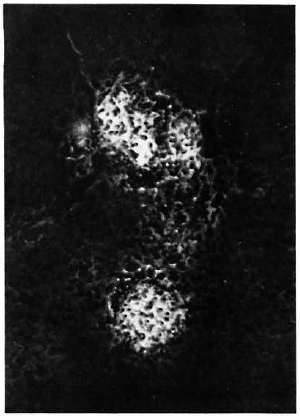
Fig. 3. Liver section from a MLM-infected mouse, stained with fluoresceinatcd rabbit anti-MLM antibody. Two medium-size lepromatoid granulomas appear neatly stained by the antibody (x 500).
A different but comparable group of 10 MLM-infected mice was examined for proteinuria 4 and 8 months after infection. Proteinuria was assessed by using diagnostic sticks for urinalysis (Miles Laboratories, Mexico). No proteinuria was detected, not even in the animals with the longer period of infection.
The above findings indicate a lack of major kidney damage in mice with well-established lepromatoid leprosy despite the important deposition of immunoglobulins in their renal glomeruli. Several studies seeking an explanation for this apparent paradox and an ultrastructural study of the kidneys in murine leprosy are in progress.
- Oscar Rojas-Espinosa, Q.B.P., Sc.Dr.
Antonina Oltra, Q.B.P.
Patricia Arce, I.B.Q.
Patricia Méndez, Q.B.P.
Departamento de Inmunología
Escuela Nacional de Ciencias Biológicas
Instituto Politécnico Nacional
Carpió y Plan de Ayala
Col. Sto. Tomas
11340 Mexico, D.F., Mexico
- Amado González-Mendoza, M.D.
Unidad de Investigación Biomedica de Occidente
Division de Patología Experimental, IMSS
Apdo. 1-3838
Guadalajara, Jalisco, Mexico
Acknowledgments. The continuous support of Dr. S. Estrada-Parra, the technical assistance of Biol. Angela Sanchez, and the periodic supply of pathogen-free NIH mice from the Laboratorio de Salud Publica de la Secretaria de Salud, Mexico, are highly recognized. Financial help on the project "Lepra lepromatosa de las ratas y ratones; el papel de los complejos inmunes en la patogenia de la enfermedad (880345)" was from DEPI, IPN. O. Rojas-Espinosa is a Fellow from CO-FAA, IPN, and SNI, Mexico.
REFERENCES
1. Giloh, H. and Sedat. J. W. Fluorescente microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gállate. Science 217(1982)1252-1255.
2. Johnson, N. W. and Rienenstock, J. Abolition of non-specific fluorescent staining of eosinophils. J. Immunol. Methods 4(1974)189-194.
3. Kelly, J., Whelan. C. A., Weir, D. G. and Feighery, C. Removal of endogenous peroxidase activity from cryostat sections for immunoperoxi-dase visualisation of monoclonal antibodies. J. Immunol. Methods 96(1987)127-132.
4. Rojas-Espinosa, O., Gonzalez, M. A., Estrada, P. S., Ortiz, Y., Gonzalez, C. O. and Perez, S. G. Presence of soluble Mycobacterium leprae-derivedantigen in the inflammatory exudate of reactional lepromatous leprosy. Lepr. Rev. 56(1985)229-238.
5. Rojas-Espinosa, O., Vega, R., Oltra, A., Arce, P. and Nunez, A. Transitory macrophage activation in the granulomatous lesions of Mycobacterium lepraemurium-inducedlepromatoid leprosy in the mouse. Int. J. Lepr. 56(1988)428-436.
6. Tanimura, T. and Nishimura, S. Studies on the pathology of murine leprosy. Int. J. Lepr. 20(1952)83-94.
Reprint requests to Dr. Rojas-Espinosa.