- Volume 59 , Number 4
- Page: 645–8
A histopathological and immunological profile of a single lesion lepromatous leprosy (LLs)
In an effort to increase the utility of the JOURNAL in continuing medical education, in this section we welcome contributions dealing with practical problems in leprosy work. Submissions to this section will undergo minimal editorial changes and may well contain controversial points. Letters to the Editor pointing out other viewpoints are welcome.
Lepromatous leprosy is usually a generalized disease with numerous macules, plaques, or nodules distributed symmetrically all over the body. Lepromatous leprosy presenting as a solitary lesion with a high bacterial index (BI) has been reported rarely.1, 2 A patient with a single skin lesion but with characteristic histopathological and immunological features of multibacillary leprosy (LLs) is presented here.
CASE REPORT
The patient is a 70-year-old, brown-skinned, female resident of a low-endemic area for leprosy in New Delhi, India. Six months prior to clinic attendance, a single, small, raised papule appeared on the ulnar border of her left wrist. It persisted for 4 months but the patient did not consult any physician or dermatologist. The lesion began to increase gradually 2 months prior to clinic attendance. At the time of examination, it was 3x 3 cm in size, well defined, erythematous, raised, infiltrated and anesthetic to pain, temperature, and touch sensations. Neither a thickened nerve to the patch was palpable nor the left ulnar or any other superficial cutaneous nerves in the region were found thickened. The remainder of the skin was uninvolved (Fig. 1). The patient had no familial or extrafamilial contact with leprosy. She was under treatment by a cardiologist for hypertension, ischemic heart disease, and atrial fibrillation. On the basis of clinical features, the patient was initially diagnosed as a case of tuberculoid leprosy and was put on paucibacillary multidrug therapy (PB MDT). At the same time a piece of involved skin was sent for histopathological examination. A histopathological diagnosis of lepromatous (LL) leprosy surprised the clinicians. A clinical re-evaluation 1 month later confirmed that there was no other skin involvement and no loss of sensory or motor functions. Slit-skin smear examination now revealed a 4+ BI positivity from the lesion with five additional sites, i.e., both eyebrows, both earlobes, and the middle finger of the right hand, were negative for acid-fast bacilli (AFB). A second skin biopsy was taken to reconfirm the histopathological diagnosis. The initial Mitsuda reaction to armadillo-derived lepromin (WHO) was negative. To reconfirm, at 6 months post-treatment a 72-hour delayed-type hypersensitivity skin reaction evaluated by intradermal injection of sonicated armadillo-derived lepromin (kindly donated by Dr. R. J. W. Rees, of NIMR, London), which was done along with an immunological profile, was also negative.
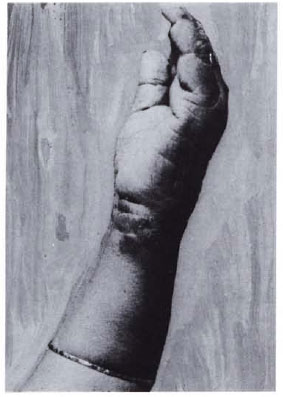
Fig. 1. Erythematous plaque 3 × 3 cm on ulnar border of left wrist.
On subsequent review and the finding of a 4+ BI from the LL lesion, the patient was put on the Government of India National Leprosy Eradication Program-modified WHO multibacillary (MB MDT) regimen, i.e., an added 600 mg of rifampin daily for 2 weeks in the first month.3
After 6 months of treatment with MB MDT, a slit-skin smear from the involved skin showed a drastic reduction in the BI from 4+ AFB before treatment to 1 + AFB. A repeat follow-up biopsy was planned on the patient a month later but, unfortunately, she was lost to follow-up.
Histopathology. Her skin biopsy shows thinning of the epidermis. The upper and mid-dermis show sheets of foamy histiocytes full of AFB on Fitc-Faraco staining. Deeper down, some lymphocytic infiltrates are seen. Within the lesion, identification of the destroyed nerves was difficult due to the intensity of the infiltrate. However, in the deeper and less-involved areas of the dermis round-cell infiltrates around the nerve twigs could be seen. There is a clear Grenz zone between the infiltrate and the epidermis (Fig. 2). The BI is 3 + .
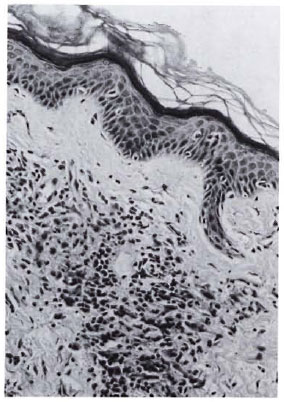
Fig. 2. Histological section showing thinning ofepidermis, clear Grenz zone, and infiltration of lepracells (hematoxylin and eosin × 250).
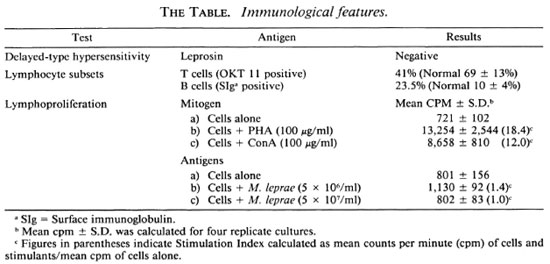
Immunology. An immunological profile done on the blood of the patient 6 months post-treatment included the following: Peripheral blood mononuclear cells were separated by Ficoll-Hypaque gradients. T and B cells were enumerated by indirect immunofluorescent staining using OKT 11 monoclonal antibody (Ortho Diagnostics) and antihuman immunoglobulin (Dakopatts) as primary antibodies. As is evident from The Table, T cells were moderately reduced; whereas B cells were mildly increased in number. To evaluate the functional status of the T cells, lymphoprolifcrative assays were undertaken using general T-cell mitogens (The Table), phytohemagglutinins (Wellcome Laboratories), and concanavalin A (Pharmacia, Ltd.) as well as specific Mycobacterium leprae antigens at two concentrations. Responsiveness to the specific antigen was poor, and mitogen-induced responses were decreased to a moderately severe degree. All of these features are similar to features expressed in multi-bacillary leprosy.4
DISCUSSION
The patient had only a single skin lesion near the left wrist with clinical characteristics of tuberculoid leprosy. Once this was refuted by histopathological, immunological, and slit-skin smear examinations, one could think of other possibilities, e.g., cultivable mycobacterium-both typical and atypical. Lupus vulgaris could present as an erythematous plaque, but this was ruled out by the inability to culture the organism in Lowenstein-Jensen (LJ) medium at 37ºC from the tissue, by the presence of a 4+ BI in the slit-skin smear, which is a rarity in lupus vulgaris5 and, in addition, by the presence of a granuloma with foamy histiocytes laden with AFB on histology.
Atypical mycobacteria, such as M. marinum, which causes indolent granulomas in hands and feet called swimming pool granulomas, and M. kansasii, which causes granulomatous lesions and subcutaneous abscesses, were ruled out by the failure of efforts to grow the organism in LJ culture medium at optimum temperature and by the lack of a distinctive granuloma on histology. Further, a shift in the spectrum of the disease histologically after only 1 month of PB MDT makes it highly unlikely that this is any acid-fast bacillus other than M. leprae.
It is of significance that no antigen-induced responses were detectable by in vivo skin testing or in vitro lymphoproliferative assays. The general T-cell numbers and functions were also decreased. These features are similar to those observed in MB leprosy patients of borderline lepromatous/ lepromatous (BL/LL) leprosy. The presentation is unusual in the sense that a lesion clinically resembling polar tuberculoid leprosy shows features of MB leprosy histologically. The histopathological identification and immunological investigations (lepromin skin test and blood profiles), if possible, are of importance in such patients.
In conclusion we can only speculate as to what factors may be involved in a situation wherein a lepromin-negative patient is able to limit the disease to a single lesion in which the bacilli had multiplied to a level of 4 + . Could it be direct inoculation of bacilli coupled with repeated trauma and low skin temperature? Surprisingly, another feature during recovery was the rapid bacterial clearance from 4+ BI (before treatment) to 1 + BI in just 6 months post-treatment on slit-skin smear examination. Since such rapid clearance of bacilli is unusual in any individual with immunological features of MB leprosy, could some local factors be responsible in addition to drug therapy for the above features and also in limiting the disease to one site only?
Leprosy remains a disease endemic to underdeveloped countries with many constraints in field conditions where it is not possible to do elaborate histopathological and immunological tests. Nevertheless, to safeguard against missing the diagnosis of multibacillary leprosy every case should be subjected to a slit-skin smear examination if possible.
- Radhey Shyam Misra, M.D.
Dermatologist and Head
- Subramanyam Ravi, D.V.&D.
Research Officer (ICMR)
Department of Dermatology and Leprology
- Bhanu Iyengar, M.D., Ph.D.
Deputy Director
Institute of Pathology (ICMR)
Safdarjang Hospital
New Delhi 110029, India
-Indira Nath, M.D., M.R.C.Path., F.A.Sc.
Professor
Biotechnology Division
All India Institute of Medical Sciences
New Delhi 110029, India
1. Job, C. K., Kahkonen, M. E., Jacobson, R. R. and Hastings, R. C. Single lesion subpolar lepromatous leprosy and its possible mode of origin. Int. J. Lepr. 57(1988)12-19.
2. Yoder, L. J., Jacobson, R. R. and Job, C. K. A single skin lesion-an unusual presentation of lepromatous leprosy. Int. J. Lepr. 53(1985)554-558.
3. Leprosy: Multi-drug Regimen Advised for Treatment of Leprosy Cases. Directorate General of Health Services (Leprosy Division). Indian Ministry of Health & Family Welfare. New Delhi: Government of India, 1985.
4. Nath, I., Curtis, J., Sharma, A. K. and Talwar, G. P. Circulating T cell numbers and their mitogenic potential in leprosy, correlation with mycobacterial load. Clin. Exp. Immunol. 29 (1977) 393-396.
5. Ramesh, V., Misra, R. S. and Jain, R. K. Secondary tuberculosis of skin: clinical features and problems in laboratory diagnosis. Int. J. Dermatol. 26(1987)578-581.