- Volume 59 , Number 4
- Page: 649–51
Antileprotic effect of the immunostimulating drug RACA 854 in experimentally infected armadillos
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
To the Editor:
The drug coded RACA 854 (formerly CID 85) is an especially manufactured natural substance used in the treatment of rheumatoid arthritis. It contains amino acids, peptides and enzymes. It possesses immunostimulating properties demonstrated in significant increases in IgG and IgM and in the stimulation of precursors of T lymphocytes to differentiate into mature T cells (7, 8). Until now, most of the antileprotic drugs used are directed against Mycobacterium leprae, the causative agent of leprosy. In this experiment, the immunostimulating properties of RACA 854 were tested against leprosy in experimentally infected nine-banded armadillos (NBA).
A total of 12 NBA were experimentally infected in the right femoral vein with a 1-ml suspension containing 5 X 108 armadillo-derived M. leprae (ADMLE). The verification of the ADMLE was made with biochemical methods (positive dopa-oxidase, dccolorization with pyridine4, 6), with immunological methods (binding with specific monoclonal antibodies directed against phenolic glycolipid-I2), and by the determination of the sequences of 16S rRNA using a reverse transcriptase (9). To exclude a mixed infection with mycobacteria other than M. leprae, a cultivation on media recommended by Portaels (5) was done with negative results.
Eighteen months after inoculation, eight NBA developed lepromas at the site of inoculation. The size of the lepromas varied from 10 mm to 20 mm in diameter (The Table). Four infected animals (nos. 1, 2, 3, and 4) were treated with 0.5 ml of RACA 854 intramuscularly three times weekly. Each animal received a total of 20 doses. Animal no. 5 was treated for 3 months with 2 ml of RACA 854 three times weekly. In addition, three animals (nos. 6, 7, and 8) were used as controls and received 0.5 ml of 0.9% NaCl solution as placebo.
From all of the animals 9 ml of blood was collected under anesthesia with 1.6 ml Ketavet® and 0.4 ml Rompun® before and at the end of treatment and 1 month later. The efTect of RACA 854 was monitored clinically until 5 months after treatment by measuring the size of the leproma at the injection site, by the occurrence of further cutaneous lepromas, and in animal no. 5 by histopathological and electron-microscopical examinations of the biopsy taken from the scar following the leproma. The blood examinations consisted of blood sedimentation; total count of leukocytes and lymphocytes; total hemoglobin content; IgG, IgA, IgM, and IgE levels; GOT; GPT; uric acid and creatinine serum concentrations.
The RACA 854 therapy in the NBA resulted in a decrease of the leproma size to a scar in 3 of 5 treated animals (Figs. 1A and 1B). In one animal the leproma diminished during treatment from 20 mm to 10 mm in diameter, showed the same size 1 month later but enlarged to 25 mm after several more months. In another treated animal there was no diminution of the leproma during treatment, and it enlarged during the 5 months after treatment (The Table). Of the untreated controls, all three animals had an enlargement of their lepromas (The Table). Furthermore, one animal had to be killed due to systemic leprosy with multiple lepromas, liver enlargement, and a spleen containing high concentrations of M. leprae.
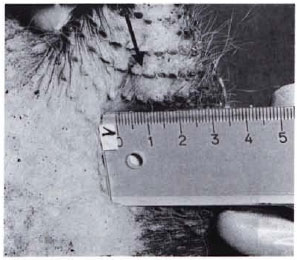
Fig. 1 A. Leproma (arrow) at the site of intravenous inoculation which developed within 18 months. Original size (1 cm diameter) before treatment with RACA 854.
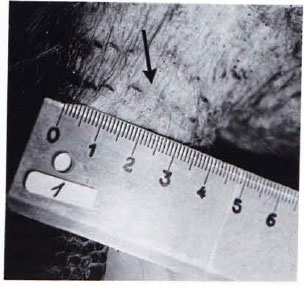
Fig. 1B. Diminution of leproma in Fig. 1A to a scar (arrow) following treatment with RACA 854 (20 intramuscular injections within 7 weeks).
In the biopsy of the leproma which had involuted to a scar in NBA no. 5, a low concentration of acid-fast bacilli (AFB) (1.5 × 106/g) with a viability of 30% (average) was found using the fluorescein diacetatc/ ethidium bromide method (3). These AFB were viable and multiplied in the foot pads of nude mice, where they caused swelling typical of experimental leprosy. These AFB were positive in the indirect immunofluorescence tests using M. leprae-specific monoclonal antibodies. The histopathological examination of the scar tissue revealed a granuloma containing AFB. By electron microscopy these bacilli appeared to be solid, intact cells (Fig. 2).
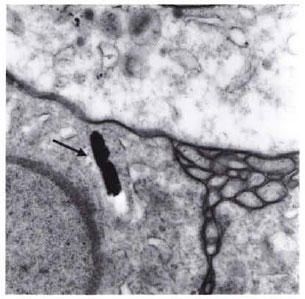
Fig. 2. In scar tissue (involutional cutaneous leproma) which developed after therapy with RACA 854, the remaining structure seen (arrow) resembles M. leprae (× 53,200).
The blood examination revealed a considerable increase in IgM and a slight increase in IgG during the RACA treatment. Furthermore, the lymphocyte count increased significantly from 13.05 ± 1.28 (mean percent ± S.D.) to 19.38 ± 4.64 at the end of the treatment.
The lymphocyte count increased in the two animals in which the leproma diminished to a scar. No side effects were observed during the RACA treatment. In the nontreated control group there were no significant differences in IgM or IgG levels or in the lymphocytic count. The leukocytic count decreased in two animals.
RACA 854 showed considerable antileprosy effect, which resulted in the regression of cutaneous lepromas to scars in 3 of 5 animals within 7 weeks of treatment. In the scar examined in animal no. 5, the concentration of leprosy bacilli was low (1.5 × 106/ g) compared with lepromas of nontrcated animals where concentrations of 1010/g to 1011/g were common (1). At least some of these bacilli were viable, causing leprosy in nude mice. Thus, the immunostimulating effect alone did not lead to the total elimination of viable leprosy bacilli in the lepromas in spite of reducing the size of the lesions and the number of bacilli.
Nevertheless, it can be expected that RACA 854 would contribute to the effectiveness of leprosy drug therapy when used together with antileprotic drugs. The experiments will be continued using multidrug therapy combined with RACA treatment of nine-banded armadillos in different stages of the experimental leprosy infection. The fact that the tolerance of RACA is good in humans opens the possibility of using its immunostimulating effect for the improvement of drug therapy in leprosy patients.
- Jindrich Kazda, Ph.D.
Professor
Borstel Research Institute
Institute for Experimental Biology and Medicine
Parkallee 22
D-2061 Borstel, Germany
- Ahmed Shaftk, M.D.
Professor
Faculty of Medicine
University of Cairo
Cairo, Egypt
Acknowledgment. The authors gratefully acknowledge that they owe the initiative of this research to Mrs. Margot Yehia, Head of the Scientific Office of Professor A. Shafik and President of the Cairo Lioness Club Leprosy Committee, who proposed and coordinated it. This study was supported by a grant from the German Leprosy Relief Association, Wurzburg. We would like to thank Mr. Werner Mohr, Mrs. Corina Dicrfeld, and Mrs. Elke Link for their skillful technical assistance.
REFERENCES
1. KAZDA, J. A more malignant course of leprosy infection in armadillos after incubation with sonicated suspension of Mycobacterium leprae. (Letter) Int. J. Lepr. 54(1956)129-131.
2. KOLK, A. H. J.; Ho, M. L., KLATER, P. R., EGGELTE, T. A., KUIJPER, S., DE JONGE, S. and VAN LEEUWEN, J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis. M. bovis (BCG) and M. leprae. Clin. Exp. Immunol. 58(1984)511-521.
3. KVACH, J. T. and VERAS, J. R. A fluorescent staining procedure for determining the viability of mycobacterial cells. Int. J. Lepr. 50 (1982) 183-192.
4. MCCORMICK, G. T. and SANCHEZ, R. M. Pyridine cxtractability of acid-fastness from Mycobacterium leprae. Int. J. Lepr. 47(1979)495-499.
5. PORTAELS, F. and PATTYN, S. R. Bacteriological studies of armadillo livers infected with Mycobacterium leprae. Ann. Soc. Belg. Med. Trop. 62(1982)233-245.
6. PRABHAKARAN, K. A rapid identification test for Mycobacterium leprae. (Letter) Int. J.Lepr.41(1973)121.
7. SHAFIK, A., EL-TAIB, S., MAHRAN, S., DARWISH, M., HAMED, A., KANDIL, A. and MAHDI, K. A contribution to the effect of CID 85 on humoral immunity. J. Drug Res. Egypt 17(1987)83-86.
8. SHARK, A., EL-TAIB, S., MAHRAN, S., DARWISH, M., HAMED, A., KANDIL, A. and MAHDI, K. The hemopoietic immunomodulating effect of CID 85. J. Drug Res. Egypt 17(1987)253-259.
9. SMIDA, J., KAZDA, J. and STACKEBRANDT, E. Molecular genetic evidence for the relationship of Mycobacterium leprae to slow-growing pathogenic mycobacteria. Int. J. Lepr. 56(1988)449-454.