- Volume 59 , Number 4
- Page: 649–51
Renal alterations in murine leprosy
To the Editor:
In 1988, a paper describing a patient with borderline lepromatous (BL) leprosy and erythema nodosum leprosum (ENL) that showed mesangial proliferative glomerulonephritis secondary to the deposition of circulating immune complexes (CIC) and epithelioid granulomas free of acid-fast bacilli (AFB) in the renal interstitium was published (1). On reviewing the literature, the authors of that paper did not find any report of AFB-positive granulomas in the kidney, although they found some reports of cases with renal granulomas with AFB (8,10). Although it had been noticed already (3,6), the above paper emphasizes the particular resistance of the renal tissue to infection by Mycobacterium leprae. The reason for this higher resistance to mycobacterial infections in general is not obvious, but it has been related to the high amount of spermine and spermidine in renal tissue (4, 5) and to the relative lack of macrophages in the renal interstitium.
In murine leprosy, despite the fact that it is a systemic mycobacteriosis, the renal tissue is, as in human leprosy, seldomly affected. While the liver, spleen, skin, lymph nodes, and other target structures are extensively parasitized in the advanced disease, the renal parenchyma remains virtually free of infection. Bacilliferous granulomas may appear, however, at the hilar zone and on the renal capsule (Figs. 1 and 2). Because of this peculiar resistance of the renal tissue to infection by M. lepraemurium (MLM), it was noteworthy to find discrete, isolated, bacilliferous granulomas (or clumps?) in a couple of glomeruli in 2 out of 10 albino NIH mice with an advanced (6 months) MLM-infection. This contrasted with the large proportion of granulomas found in the target organs of all of the examined animals. Thus, renal tissue in the mouse seems to be as resistant to infection by MLM as human renal tissue is to infection by M. leprae.
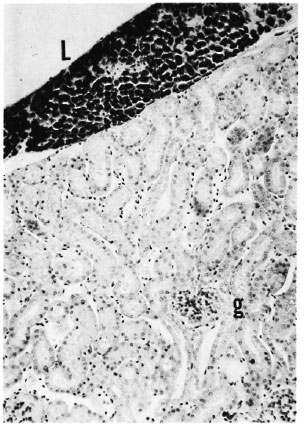
Fig. 1. A highly bacilliferous leproma (L) associated with the renal capsule of a mouse with more than 6 months of MLM infection. Despite the advanced stage of the infection, no granulomas or other major alterations are seen in the renal interstitium nor in the three glomeruli (g) shown (Ziehl-Neelsen and H&E × 100).
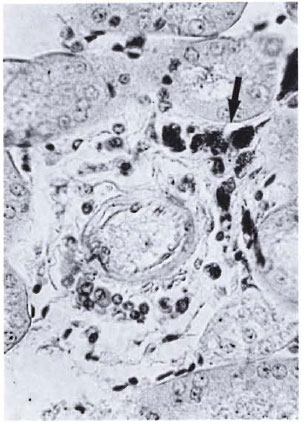
Fig. 2. Bacilliferous granulomas of moderate size (arrow) frequently seen around blood vessels of the hilar zone in the kidneys of mice with advanced MLM infection. Beyond this region, isolated bacilli or bacilli-containing granulomas are rarely found (Ziehl-Neelsenand H&E × 400).
The other aspect of the paper by Al-Mohaya, et al. (1) refers to the development of glomerulonephritis in their lepromatous patient. Glomerulonephritis, one of several renal lesions that have been described in human leprosy, although relatively common is not a general complication of the disease. Most frequently, it has been observed in those lepromatous cases with recurrent leprosy reactions (2), and it has been related to the chronic deposition of CIC. In patients with long-lasting disease, however, CIC involve mycobacterial antigens, but they also involve self-antigens. Thus, glomerulonephritis may result from the deposition of mycobacterial-related CIC, but it may also result from the deposition of other microbial-rclatcd CIC (from associated infections), from the deposition of self-related CIC, or as a result of autoantibodies reacting with glomerular structures, since a variety of autoantibodies have been reported in lepromatous leprosy (11). Therefore, since multiple etiologies may give rise to CIC in leprosy (and these, in turn, may lead to diverse renal lesions), it is not clear to what extent leprosy itself accounts for the renal pathology reported in the literature (for a short, comprehensive review see 7).
Murine leprosy (a disease similar in some aspects, different in others, to human leprosy), on the other hand, being susceptible to all sorts of controls (homogeneity in sex, age, and weight of the host population; knowledge of the exact infecting doses and time of infection; exclusion of other associated infections; and adequate housing and nourishment), is appropriate for the study of renal pathology resulting from a chronic mycobacterial disease. In the murine infections, abundant antibodies to mycobacterial antigens, hence very likely CIC, are produced. CIC that deposit on glomerular structures give rise to lesions that do not involve the participation of polymorphonuclear (PMN) leukocytes and, hence, do not become destructive. The thickening of the mesangial matrix (PAS and Mallory stains), hypertrophy of the very proximal renal tubules that alters the morphology of the Bowman's capsule giving the impression of glomerular retraction, and the appearance of an amorphous granular material filling up both the enlarged subcapsular space and the lumen of the adjacent proximal renal tubules, are the most frequent anomalies noticed (Fig. 3). Amyloid (methyl-violet and Congo red stains) was not a component of the glomerular lesion.
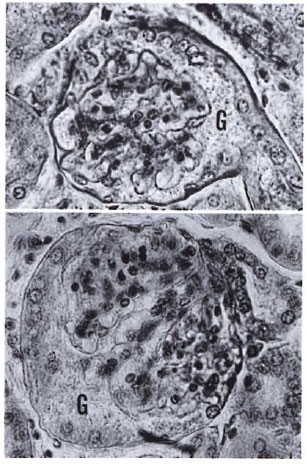
Fig. 3. Glomeruli in murine leprosy often present nondestructive alterations such as increases in volume, hypertrophy of the connecting proximal tubules (notice nucleated cell layer lining the glomerular capsule), and the presence of amorphous granular material (G) filling up the increased subcapsular space (H&E × 400).
The lack of tissue destruction, even in the presence of immune complexes (we have previously demonstrated the heavy deposition of IgG and IgM immunoglobulins in the glomeruli of mice with advanced murine leprosy; Figures 4 and 5), is probably due to the lowered complement activity which, in turn, might account for the absence of PMN infiltration. The low complement activity observed in the infected animals seems to be due to the production of a certain factor that very likely inhibits activation of cither CI, C4, or C2 since the alternative route remains almost unaffected (Rojas-Espinosa, et al., submitted for publication). This inactivation of complement that appears and increases during the course of the infection minimizes the general tissue damage and delays the eventually lethal outcome of the disease, allowing the bacilli to preserve their host longer. We do not know whether this is a particular characteristic of murine leprosy or if it also happens in human leprosy, although here this situation might be masked by pathological factors secondary to the mycobacterial disease.
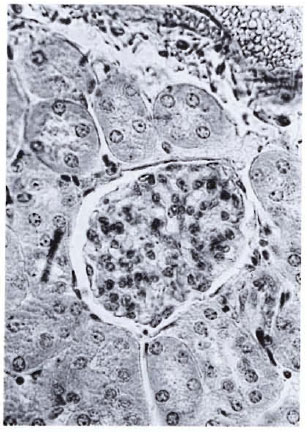
Fig. 4. A normal glomerulus showing average sizeand cellularity, the neat Bowman's capsule, and clearsubcapsular space (H&E × 400).
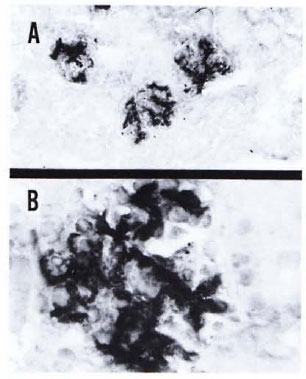
Fig. 5. A kidney section from a mouse with 7 months of MLM infection showing a heavy deposition of IgG. A similar but less-intense reaction was observed when the renal section was reacted with an antibody to mouse IgM. This particular specimen showed negligible deposition of murine C3 (Immunoperoxidase stain × 100 (A) × 400 (B).
- Oscar Rojas-Espinosa, Q.B.P., Sc.Dr.
Departamento de Inmunología
- Elba Reyes Maldonado, Q.B.P.
Departamento de Morfología
Escuela de Ciencias Biológicas
Instituto Politécnico Nacional
11340 México, D.F., México
Acknowledgment. This study was supported by the Dirección de Estudios de Posgrado e Investigación, Instituto Politécnico Nacional, México. O. Rojas-Espinosa is a fcllowholdcr from the COFAA, IPN, and SNI, Mexico.
REFERENCES
1. AL-MOHAYA, S. A., COODE, P. E., ALKHDER, A. A. and AL-SUHAIBANI, M. O. Renal granuloma and mesangial proliferative glomerulonephritis in leprosy. Int. J. Lepr. 56(1988)599-602.
2. DRUTZ, D . J. and GUTMAN, R. A. Renal manifestations of leprosy: glomerulonephritis-a complication of erythema nodosum leprosum. Am. J. Trop. Med. Hyg. 22(1973)496-502.
3. GUPTA, J. C., DIWAKAR, R., SINGH, S., GUPTA, D. K. and PANDA, P. K. A histopathologic study of renal biopsies in fifty cases of leprosy. Int. J. Ixpr. 45(1977)167-170.
4. HIRSCH, J. G. The essential participation of an enzyme in the inhibition of growth of tubercle bacilli by spermine. J. Exp. Med. 97 (1953) 327-344.
5. HIRSCH, J. G. Spermine oxidase; amine oxidase with specificity for spermine and spermidine. J. Exp. Med. 97(1953)345-355.
6. IVESON, J. M., MCDOUGALL, A. C, LEATHAM, A. J. and HARRIS, H. J. Lepromatous leprosy presenting with polyarthritis, myositis, and immune complex glomerulonephritis. Br. Med. J. 3(1975)619-621
7. NG, W. L., SCOLLARD, D. M. and HUA, A. Glomerulonephritis in leprosy. Am. J. Clin. Pathol. 76 (1981) 321-329.
8. POWELL, C. S. and SWAN, L. L. Leprosy: pathologic changes observed in 50 consecutive necropsies. Am. J. Pathol. 31(1955)1131-1147.
9. ROJAS-ESPINOSA, O., OLTRA, A., ARCE, P. and MENDEZ, P. Glomerular immunoglobulin deposition in the absence of tissue damage in murine leprosy. Int. J. Lepr. 57(1989)879-882.
10. SAINANI, G. S. and RAO, K. V. N. Renal changes in leprosy. J. Assoc. Physicians India 22(1974)659-664.
11. WAGER, O. Immunological aspects of leprosy with special reference to autoimmune disease. Bull. WHO 41(1969)793-804.
New JOURNAL Address
Effective 15 January 1992, the new address for the JOURNAL'S Editorial Office will be:
INTERNATIONAL JOURNAL OF LEPROSY
P.O. Box 25072
Louisiana State University
Baton Rouge, Louisiana 70894, U.S.A.
Robert C. Hastings, M.D., Ph.D.
Editor
Dee D. Goodman, B.A
Assistant Editor