- Volume 59 , Number 4
- Page: 658–69
News and Notes
This department furnishes information concerning institutions, organizations, and individuals engaged in work on leprosy and other mycobacterial diseases, and makes note of scientific meetings and other matters of interest.
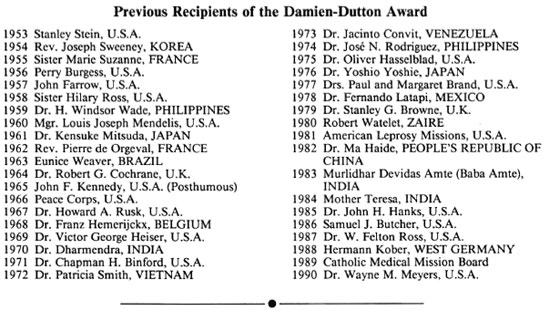
1991 DAMIEN-DUTTON AWARD RECIPIENT
Dr. Ruth K. M. Pfau

Dr. Ruth Pfau receives the 1991 Damien-Dutton Award from Dr. Wayne Meyers, the 1990 Award winner.
From Karachi to Azad Kashmir, for 30 arduous years, leprosy is her mission. Beginning as a young doctor, a vowed religious of the mission-sending Society of the Daughters of the Heart of Mary, Dr. Ruth Pfau arrived in Pakistan in 1960 to work with her community in their social programs. But she was not long in settling on what was to be her life work. The 15' x 15' x 15' site in Karachi where the work was started is today the famed Marie Adelaide Leprosy Center-cum-Hospital with 86 beds and 8000 outpatients, with subcenters in Karachi and 40 field clinics in outlying provinces, treating some 32,000 afflicted.
Most outstanding'is her drive for complete rehabilitation, providing education and professional training to enable the discharged to become self-supporting. More than 60% of its former patients are employed in the Center throughout the hospital and its far-flung clinics.
Dr. Pfau's contribution over the past 30 years has been wide and varied, with a lasting impact on world-wide leprosy endeavor. A born teacher and organizer, she early commenced training leprosy technicians, with some 325 now the backbone of the National Leprosy Control Program of Pakistan.
As a young girl of 15 in Leipzig at the time of Germany's liberation from Hitler and his regime, she found further restraints when her country was divided. She was the first child in her family to escape to the West. With freedom there came the need to choose from all the opportunities opening up to her. Ever open to the needs of others, and drawn to the ageless beauty of God in all, she elected to devote her life to working as a doctor to alleviate human misery and to restore human dignity.
Dr. Pfau has received innumerable honors. In 1969 she received the German Bundesverdienstkreuz and in the same year, was awarded the Pakistani Sitara-i-quaid-e-Azam. In 1978 she received another high German award-the Grosse Bundesdienstkreuz-and the same day she received Pakistan's Hil-e-Imitiaz. In 1980 Dr. Pfau was appointed Honorary Advisor on Leprosy to the Government of Pakistan. In 1985, in recognition of her 25 years of service to leprosy work in Pakistan, the Federal Republic of Germany awarded her with the Grosse Verdienstkreuz mit Stern. In March 1989, Dr. Pfau was granted an honorary Pakistani citizenship by its President, who also awarded her with their highest award, the Hilal-i-Pakistani.
France. Diplôme Universitaire de Lépologie, 1991-1992. Un enseignement théorique et pratique de léprologie sera organisé à la Faculté de Médecine Lariboisière-Saint-Louis au cours de l'année universitaire 1991-1992 sous la direction du Professeur F. Cottenot. Cet enseignement théorique, clinique et de laboratoire aura lieu, dans le service de Dermatologie du Professeur Dubertret à l'Hôpital Saint-Louis et à la Faculté de Médecine Lariboisière-Saint-Louis. Il débutera le mardi 7 janvier 1992 et se terminera fin mars 1992.
Seront admis à suivre l'enseignement: 1. Les docteurs en médecine français. 2. Les étrangers pourvus du Diplôme français de doctorat d'université. 3. Les étrangers possédant un Diplôme médical permettant l'exercice de la médecine dans leur pays d'origine.
Pourront être admis également les internes en médecine des CHR, et les étudiants des UER médicales ayant validé leur stage pratique, mais le certificat ne pourra leur être délivré que lorsqu'ils auront le diplôme de docteur en médecine.
INSCRIPTIONS: avant le 1er novembre 1991 au bureau des Spécialités médicales Faculté; Villemin, 10, avenue de Verdun, 75010 Paris, France. Tél. 42 03 94 26.
Pour tous renseignements complémentaires: s'adresser au Secrétariat du Cours: Docteur Flageul, Service du Professeur Dubertret, Hôpital Saint-Louis, 1, avenue Claude-Vellefaux, 75475 Paris CEDEX 10, France. Tél. 42 49 98 16.
India. Birla Award to Dr. Dastur. Dr. D. K. Dastur, director of the Bombay Hospital's department of neuropathology and applied biology, was presented with the 11th Rameshwardas Birla National Award 1991 for his outstanding work in clinical and experimental neuropathology. The award was presented by Mr. C. Subramaniam, governor of Maharashtra, in Bombay on 21 February 1991. Dr. Dastur has made significant contributions, publishing over 180 scientific papers and editing one book each on leprosy, neurotuberculosis and neurological sciences. We extend to him our congratulations.-RCH
Comparative leprosy vaccine trial launched in South India. On 30 January 1991, Honorable Justice (retired) Mr. Krishnaswamy Reddy launched the ICMR comparative leprosy vaccine trial in village Malaipattu in Chengai-Anna district of Tamil Nadu. It was fittingly conducted on Martyrs day which is also antileprosy day and was observed as a solemn occasion. Dr. H. Srinivasan, President, Indian Association of Leprologists; Dr. V. Ekambaram, former State Leprosy Officer; (Tamil Nadu), Dr. Gangadhar Sharma, Hindu Mission Hospital, (Tambaram); Deputy Director of Public Health & Preventive Medicine (on behalf of Director of Public Health), Tamil Nadu; Shri G. Srinivasan, Member, Ethical Committee; Dr. J. A. Ponniah, Director, SLRTC, Karigiri; Dr. A. Dutta, Director, CLTRI, Chingleput; Dr. S. Basu, Director, BCG Vaccine Laboratory, Madras; Dr. P. N. Neelan, former Director, CLTRI; Dr. S. Radhakrishna, Director, IRMS (Madras); and Dr. V. P. Bharadwaj, Officer-in-Charge, CJIL, Agra, were some of the dignitaries present during the occasion. About 300,000 people from Chengai-Anna district will be participating in this vaccine trial in which three different candidate vaccines-ICRC, Mycobacterium w. and BCG in combination with killed M. leprae-will be compared for their prophylactic efficacy against leprosy. Intake for this trial is expected to continue for about 2 years, and the first results are expected after 6 years. The trial may continue for about 10 years. The trial is being conducted by the CJIL Field Unit of the Indian Council of Medical Research under the leadership of Dr. M. D. Gupte with full support from the government of Tamil Nadu.-Indian J. Lepr. 63 (1991) 140-141
Conference of Voluntary Leprosy Institutions of Maharashtra. The first Conference of Maharashtra State Voluntary Leprosy Institutions organized by the National Leprosy Organization (NLO) and hosted by Kushtarog Niwaran Samiti, Wakadi, was held at Panvel during 13-15 December 1990. It was attended by 27 representatives of voluntary organizations working in the field of leprosy in the state. Besides the inaugural and concluding sessions there were four working sessions. The role of voluntary agencies, ways to enlarge public participation, need for training and re-training, and problems faced by voluntary agencies were the four themes discussed in the working sessions. In the inaugural session, Shri S. P. Tare, Secretary, NLO, explained the objectives of the Conference and the relevance of the themes for discussion.-Indian J. Lepr. 63 (1991) 136-137
Evaluation of an MDT workshop. A Workshop on MDT of Leprosy in Low-endemic Areas: An Evaluation was organized by the Department of Dermatology and Leprology, Safdarjang Hospital, New Delhi, from 19-22 November 1990. Program managers at various levels, viz., heads of departments of dermatology in medical colleges, state and district leprosy officers from northern and northeastern states, and health personnel manning peripheral health care services like medical officers working in central government health scheme, employees state insurance corporation, Delhi administration, voluntary agencies and autonomous bodies participated in the Workshop. The objectives of the Workshop were to evaluate the state of leprosy care available, to define the existing lacunae, to help peripheral level workers to update their knowledge about the disease, and to recommend to the government suitable measures to achieve the goal of the NLEP by 2000 A.D. - Indian J. Lepr. 63 (1991) 132-135
Indian Journal of Leprosy permanent address. The "Indian Journal of Leprosy" has now been established permanently at the following address:
Dr. H. Srinivasan
Honorary Editor
Indian Journal of Leprosy
245 T. T. K. Road
Madras 600018, India
Largest vaccine trial yet. The third and largest vaccine trial yet was started officially on World Leprosy Day 1991. It is taking place in an area just to the west of Madras in Tamil Nadu, India.
Over the next 3 years a population of 300,000 will be vaccinated. Different cohorts will receive BCG, killed M. leprae at the same concentration used in the Venezuela trial, a placebo, Mycobacterium w, or the ICRC vaccine. The last two have been developed as possible prophylactic agents in India.
The study is led by Dr. Gupte, an Assistant Director of the Central JALMA Institute for Leprosy Research, Agra. It is entirely organized and funded by the Indian Council for Medical Research.
The first trial of a possible leprosy vaccine was headed by Dr. Convit in Venezuela. It involved the vaccination of some 30,000 household contacts of known patients in the period 1984-1986. The codes will be opened and the first results made known in September 1991.
The second trial received support from ILEP Members. It involved the vaccination of the total population of the Karonga District of Malawi, 125,000 people, during 1986-1989. First results are expected in 1996.
Scientists of the WHO Working Group on Leprosy Control in July 1991 pointed out that despite the optimism engendered by the success of MDT, the search for a vaccine or immunodiagnostic agent continues to be important. No disease has yet been eradicated by effective treatment alone, without some means of prevention. Even if the target of public health control by the year 2000 is achieved, there may well be a need for preventive action in at-risk populations.-ilep flash 5 (1991) 2
"Leprosy-Diagnosis and Management" 4th edition. The fourth edition of the revised and updated version of the book "Leprosy-Diagnosis and Management" written by Dr. C. K. Job, the famous pathologist; Dr. A. J. Selvapandian, former Head of the Reconstructive Surgery Unit in C.M.C. Hospital, Vellore; and Dr. C. K. Rao, former DDG (Lep), Government of India; is under publication. The book contains 23 chapters covering history, definition, epidemiology, etiology, pathogenesis and pathology, skin smears, immunology, classification, diagnosis, clinical features, treatment, reactions, eye lesions, ear, nose and throat lesions, peripheral neuritis, visceral lesions, prevention and control, deformities, reconstructive surgery, occupational therapy, plantar ulcers, surgical management in control program, and rehabilitation in leprosy, with 62 illustrations. The book will be useful to medical officers, paramedical workers, and other nonmedical personnel working in leprosy. The book is priced at: Paperback edition: Inland, Rs. 40/- Foreign, £3/- (equivalent in US $); Hardbound edition: Inland, Rs. 50/- Foreign, £4/- (equivalent in US $). Packing and forwarding charges (Rs. 10/- in India and £1 or $2 in foreign countries) will be extra. To order book, contact:
Hind Kusht Nivaran Sangh
1 Red Cross Road
New Delhi 110001, India
-Kusht Vinashak 13 (1991) 31
XVII Indian Association of Leprologists Conference. The next Biennial Conference of the Indian Association of Leprologists (IAL) will be held at Durg-Bhilai (Madhya Pradesh)-2-4 January 1991. It will be followed by a 1-day Post-Conference Workshop on "Advances in Laboratory Techniques with Reference to Patient Care" on 5 January 1992. The venue of the conference and workshop will be Kala Mandir/ Bhilai Hotel, Bhilai. Addresses for the Organizing Committee and the Scientific Committee are:
Dr. P. R. Mangalani
Organizing Secretary
XVII Biennial Conference & Workshop of IAL
Leprosy Eradication Unit Camp 1
Near New Basant Cinema, G.E. Road
Bhilai, Durg Dist. (M.P.), IndiaDr. V. P. Bharadwaj
Scientific Secretary
XVII Biennial Conference of ILA
Central JALMA Institute for Leprosy
Taj Ganj, Agra 282001 (U.P.), India
Dr. V. M. Katoch, Honorary IAL Secretary, will be the Convenor of'the Post-Conference Workshop. His address is:
Dr. V. M. Katoch
Convenor
XVII Biennial Conference of IAL
Central JALMA Institute for Leprosy
Taj Ganj, Agra 282001 (U.P.), India
-Kusht Vinashak 13 (1991) 24
Southern regional IAL conference on MDT. A Southern regional conference of the Indian Association of Leprologists (IAL) on multidrug therapy (MDT) was held at Sri Venkateshwara Medical College, Thirupathi, during 21 and 22 January 1991. The Conference was hosted by the Italian Leprosy Association (Amici di Raoul Follereau). Members of IAL in the southern states of Andhra Pradesh, Karnataka, Kerala, Pondicherry, and Tamil Nadu and district leprosy officers involved in implementation of MDT in these states, staff of all the centers aided by the Amici di Raoul Follereau, and a few resource persons from outside the region were the invitees. In all, 92 delegates including 68 district leprosy officers attended the Conference. There was an inaugural session, followed by six scientific sessions and a valedictory session.- Indian J. Lepr. 63 (1991) 138-139
"Wall Journal" on leprosy at Sion Medical College. In fulfillment of its commitment to the Bombay Municipal Corporation to augment education with reference to leprosy in the medical colleges, Bombay Leprosy Project (BLP) initiated a new experiment in medical communication by putting up a "Wall Journal" on leprosy in the campus of the Preventive and Social Medicine (PSM), LTM Medical College and Sion Hospital, Sion, Bombay. The Extension Center of BLP is already functioning. Dr. R. Ganapati, Chief Editor of the journal and Director of the Bombay Leprosy Project, in his editorial stated that this novel venture is meant to update the staff and students on current developments in leprosy.
At the function held on 2 July 1991 to mark the inauguration of the journal, Dr. Ganapati reiterated his statement that the war against leprosy should be fought from general medical institutions and not from leprosy hospitals. Dr. (Mrs.) S. S. Deshmukh, Hon. Advisor of the journal and Dean of the College, welcomed the efforts on the part of BLP wholeheartedly on the college premises since staff and students generally do not have access to leprosy literature. Dr. P. S. N. Reddy, Associate Editor and Professor and Head of PSM Department of the College, made a reference to the contribution of BLP to the department as significant. Mr. P. V. Purandhare, BLP Public Relations Officer, proposed that this journal can become more broad based if all city doctors from municipal and government hospitals come forward to share their views with voluntary organizations like BLP. - Materials received from R. Ganapati
Kenya. International Congress for Infectious Diseases, 1992. The Kenyatta International Conference Centre in Nairobi will be the setting for the International Congress for Infectious Diseases on 7-11 June 1992. The Congress is sponsored by the International Society for Infectious Diseases in collaboration with the Kenya Medical Research Institute and the World Health Organization. The Congress' preliminary program indicates that the meeting will take the theme of "Science for Development" as it applies to infectious diseases, with particular emphasis on developing countries. The program is constructed to give in-depth analysis of selected problems with state-ofthe-art lectures on, for example, progress in strategies for vaccine development, the role of vectors in the spread of disease, and mothers, children, and infectious diseases. Symposia and oral presentations plus poster sessions will be held. Deadline for submission of abstracts is 1 February 1992. For further details contact: WKV, % Interconvention, A-1450 Vienna, Austria. Fax: (43-1) 2369-648.
Maldives. Unique prevention trial. In an attempt to demonstrate the possibility of chemoprophylaxis and zero transmission of leprosy, a special program was launched in the Maldives in March 1991. Starting with Male and 16 high-risk islands, every individual will be physically examined. All new cases of leprosy will be put on MDT while all other people with no contraindication will be given a single dose of 20 mg/kg body weight of rifampin. It is hoped that the latter will prevent development of incipient cases and stop transmission by subclinical cases. The work is done by integrated mobile teams visiting the isolated islands one by one. During a team visit, no one is allowed off the island until the survey is complete. The total population involved is 120,000. The study was suggested by Dr. Melville Christian, a previous Director of Karigiri. -ilep flash 5 (1991) 1
New Zealand. New name for Leprosy Trust Board. The Leprosy Trust Board (LTB) is changing its name to the Pacific Leprosy Foundation. The new name emphasizes their broadening focus to the wider Pacific area. Documents produced by the Co-ordination Bureau will continue using the initials LTB until the new ILEP information management system is installed. -ilep flash 5 (1991) 3
Poland. International Congress of Dermatology. The chairman of the Polish Dermatological Society announces that the 24th Congress of Dermatology will be held from September 24 through 26, 1992, in Gdansk, Poland. The program will consist of plenary sessions concerning progress in etiology, pathogenesis, diagnostics, and treatment of skin diseases. Cases concerning mycology, oncology, psoriasis, veneral diseases, immunodcrmatology, dermatosurgery, photobiology and phototherapy, and pediatric dermatology will also be presented. Poster presentations are preferred. For further information, contact Professor S. Jahłońska, Katedra i Klinika, Chorŏb Skŏrnych i Wenerycznych, ul Dębinki 7, 80-211 Gdansk, Poland.-Arch. Dermatol 127 (1991) 785
Senegal. Formation Pratique pour la Prévention des Invalidités et la Réadaptation dans la Lèpre. Second cours annuel organisé par l'Institut de léprologie appliquée de Dakar-Fondation de l'Ordre de Malte, en collaboration avec la DAHW-Sénégal.
Dates: du 6 janvier au 15 février 1992
Durée: 6 semaines
Nombre de participants: 6
Objectif: former des responsables pour la mise en oeuvre, l'organisation et le développement des techniques de réadaptation fonctionnelle, d'éducation sanitaire et de prévention des invalidités dans le cadre d'un Programme national de lutte contre la lèpre, ou dans une institution spécialisée.
Type d'enseignement: participatif et pratique.
Contenu: généralités sur la lèpre; évaluation et prise en charge des atteintes nerveuses; chirurgie de la lèpre: indications, bilans pré- et post-opératoires, rééducation fonctionnelle; prescription et fabrication de chaussures adaptées; réadaptation médicale; éducation sanitaire; conception, organisation, application et évaluation d'un Programme de réadaptation; techniques de communication et formation des auxiliaires.
Langue: français parlé couramment et écrit. Mode d'admission: sur dossier, après analyse des objectifs professionnels.
Date limite de dépôt des candidatures: 31 octobre 1991.
Condition d'admission: ne seront retenus que les dossiers des candidats bénéficiaires d'une bourse couvrant les frais de voyage et de séjour.
Niveau requis: kinésithérapeute et ergothérapeute de préférence, ou infirmier spécialiste-lèpre expérimenté.
Pour tout renseignement s'adresser à:
DR. J. MILLAN, Directeur
Institut de Léprologie Appliquée de Dakar
B.P. 11023, Dakar-CD, Sénégal
Fax: % COTOA: (221) 22 01 00
Switzerland. Global intensification of work against leprosy. The first meeting of the new WHO Working Group on Leprosy Control, held in Geneva on 1-3 July 1991, agreed that the World Health Assembly target of eliminating leprosy as a public health problem (prevalence of 1 per 10,000 or less) by the year 2000 is achievable if there is an intensification of effort and mobilization of further resources.
The Working Group is a new body which is expected to meet every 18-24 months, and exists to provide a forum to review global progress against leprosy and encourage cooperation between the various agencies concerned-governments, NGOs, and WHO. Some 25 people participated including the Chair of the Medical Commission and the General Secretary of ILEP, those responsible for leprosy from the WHO Regional offices, the Chairs of IMMLEP and THELEP, five people with experience-or responsibility for national leprosy programs, and the staff of the WHO Leprosy Unit, as well as the President of ILA, and a number of other people. The tenor of the discussions was one of optimism and of cooperation. The key word was "intensification" of action and it was clear that both ILEP and WHO increasingly think in terms of focussing attention on critical countries and areas. These are areas with high numbers of patients and/or a currently low rate of MDT implementation. On figures given by Dr. Noordeen of the WHO Leprosy Unit, 96% of all registered cases come from only 30 countries and of these India, Brazil, Nigeria, Myanmar, and Indonesia alone account for 84% of registered cases. The need is to develop and implement effective national control programs in all these countries.
There was also discussion of the desirability of greater compatibility between the global reporting systems run by the Epidemiology Unit of the Catholic University of Louvain, the WHO Leprosy Unit, and ILEP. The essential indicators proposed by the recent international meeting in Djakarta on the epidemiology of leprosy were noted. Except for the addition of relapse rates, these are largely the same as those already considered in connection with the revision of the ILEP B questionnaire.
Interestingly, although it was reiterated that leprosy services can be applied through integrated services, it was agreed that it is more important to get on with implementing MDT via whatever structure exists or is appropriate locally than to delay action while awaiting integration.
Overall, this meeting provided a practical overview of the issues to be confronted if the targets of MDT for all (ILEP) or elimination of leprosy as a public health problem (WHO) by the year 2000 are to be reached. Participants left feeling the aim was possible but that it would require a true partnership between governments in the crucial countries who must ensure effective national programs, ILEP Members, other NGOs, and WHO.-ilep flash 5 (1991) 1
ILEP celebrates 25th anniversary. On 7 June 1991, 17 antileprosy associations and over 100 supporters met together in Bern to celebrate the 25th anniversary of the International Federation of Anti-Leprosy Associations (ILEP) and to re-dedicate themselves to work in the field of leprosy. At first a federation of 11 European associations, ELEP was founded in 1966, and became ILEP in 1975 with the admission of members from North America and the Pacific region. ILEP is currently active in 96 countries. Present officers of ILEP include: President Jean Loiselle, Cardinal Léger Institute Against Leprosy, Canada; Vice-President Bill Edgar, The Leprosy Mission International, Great Britain; Past President Hermann Kober, Deutsches Aussâtzigen-Hilfswerk, Germany, and Members Dr. Wayne M. Meyers, Leonard Wood Memorial, U.S.A., and Walter Rosenfeld, Aide aux Lépreux Emmaus-Suisse, Switzerland.
ILEP working groups include: Social Aspects; Fundraising and Fiscal Issues (Chair: Follereau France); Training (nonmedical aspects) (Chair: Deutsches Aussätzigen Hilfswerk); Evaluation, Monitoring and Planning (Chair: Damien Foundation); Contacts with Third World Associations (Chair: Cardinal Léger Institut). The Coordinating Bureau is composed of Paul Sommerfeld, General Secretary, and Dominique Martineau, Assistant General Secretary, and their staff.
In their first Annual News, ILEP focused on their Medical Commission-five members elected for 4-year terms. Each Commission member has responsibility for one of five disciplines: Dr. W. Felton Ross (Training), Dr. Pieter Feenstra (Leprosy Control), Dr. M. F. R. Waters (Therapy), Professor Jacques H. Grosset (Research), and Dr. P. Bourrel (Rehabilitation). The Commission members elect their own chair, currently Dr. W. Felton Ross. ILEP's Medical Commission provides member associations with medical and technical advice of a general nature, and is able to advise on particular projects if so requested. A major link between the Medical Commission and the member associations is by the coordinators of each of the five disciplines, organizing meetings and arranging for the funding of projects developed through them. In addition the Medical Commission formally advises the member associations on technical matters through its Medical Bulletins. Three bulletins were published in 1990, and all (in both French and English) are available free from ILEP members. One of the recent bulletins, entitled Basic Requirements for Implementation of Multidrug Therapy, has made a major contribution to the debate on basic requirements for MDT with a view to getting MDT to those areas where implementation is particularly difficult.
ILEP plans to publish a yearly ILEP Annual News. - From the report
Shelf life of antileprosy drugs. Ciba-Geigy, Basel, Switzerland, has kindly supplied the following information: Defining the "shelf life" of a drug as the period between the date of manufacture and expiration date, the figures for antileprosy drugs produced by Ciba-Geigy are as follows: dapsone, 4 years; Rimactan (rifampin), 4 years; Lam¬ prene (clofazimine), 5 years. -Leprosy Review 62 (1991) 235
TDR calls for applications for support of research training. The UNDP/World Bank/ WHO Special Programme for Research and Training in Tropical Diseases (TDR), established in 1976, has two objectives: (1) research and development of new tools to control the TDR target diseases: malaria, filariasis (including onchocerciasis), African trypanosomiasis and Chagas disease, leishmaniasis, schistosomiasis and leprosy; (2) strengthening of research capabilities in countries where these diseases are endemic.
As an important way to achieve its second objective, TDR provides funding to train research workers from disease-endemic countries. TDR support enables research workers to acquire research skills related to one or more of the TDR target diseases or in a discipline related to these diseases, such as molecular and cell biology, immunology, entomology, parasitology, epidemiology, clinical pharmacology, and the social sciences. Applications relating to product development and molecular entomology are also welcome. Funding is available for opportunities in established training programs for studies leading to a doctoral level degree, or for an individualized postgraduate program in a center which conducts research in tropical diseases. Support for Masters level courses will be considered in exceptional cases.
The maximum duration of TDR support for research training is 3 years. Those eligible to apply for research training include:
staff members of (i) institutions currently receiving one of the TDR institution-strengthening grants, and (ii) other institutions where TDR support for such grants came to an end 2 to 3 years ago; scientists from other institutions who are already engaged in research or committed to doing research on one or more of TDR's target diseases, and whose home institution is equipped with required research facilities; staff members of Ministry of Health disease control services who are involved in planning, executing and evaluating disease control programs related to TDR's target diseases; scientists who have had appropriate postgraduate training in epidemiology, social sciences, and other field-oriented subjects and who require practical, hands-on (postdoctoral) training in a research project or suitable institution doing field research in one of TDR's target diseases; scientists with postgraduate research training who have been actively involved in clinical, field or laboratory research in one of TDR's target diseases for a minimum of 5 years and who now want to spend a period of time in a suitable research center or laboratory to upgrade their skills or to carry out specialized experiments or data analyses.
Address: Dr. J. A. Hashmi, Special Programme for Research and Training in Tropical Diseases, WHO, 1211 Geneva 27, Switzerland. -Lepr. Rev. 62 (1991) 349
WHO Consultation on early diagnosis of leprosy. A WHO Consultation on the early diagnosis of leprosy took place in Geneva from 23 to 25 May 1990, with the participation of experts from nine countries in the fields of clinical leprosy, histopathology, serology, and epidemiology. The Consultation reviewed all aspects of current knowledge on the early diagnosis of leprosy, including newer diagnostic tools, and considered the cost-effectiveness of control programs.
Multidrug therapy (MDT) against leprosy, first recommended by WHO 9 years ago, has achieved significant reductions in disease prevalence in several countries and has encouraged self-reporting of cases. In view of the success of MDT in the treatment of registered leprosy cases, the meeting recommended that high priority should now be given to reaching patients not yet identified. The MDT treatment strategy should be supported by improved case-detection and early, reliable diagnosis by trained personnel. Individuals reporting to clinics should be clearly categorized as having advanced leprosy, early leprosy, suspect leprosy, or no evidence of leprosy, and dealt with accordingly. Efforts should be made wherever necessary to improve the quality of skin-smear services. Histopathology is a useful additional tool for establishing the diagnosis in suspect cases, but routine histopathology of all cases may not be necessary.
The Consultation also recommended certain investigations for future research.- World Health Forum 12 (1991) 116-117
WHO Tropical Diseases Research (TDR) budgets cut. In order to fund three new initiatives requiring 25% of the overall TDR budget, all but one of the scientific steering committees suffered budget cuts by the Scientific and Technical Advisory Committee. In general, the most successful steering committee received the largest cut. Because of the apparent success of multidrug therapy, the Therapy of Leprosy Steering Committee's budget was cut substantially.-TDR news 35 (1991) 1-2
U.K. ILEP General Secretary joins governing membership of UIA. Paul Sommerfeld, General Secretary of ILEP, was invited recently to join the governing membership of the Union of International Associations (UIA). The UIA, based in Brussels, has existed for over 80 years to facilitate the evolution of associative activity at an international level. It is best known for publishing the Yearbook of International Organisations.
The UIA is governed by a membership body which can only be joined through personal invitation and in an individual capacity. It is limited to a maximum of 120 and includes a global cross-section of people involved in international affairs such as politicians, academics, and officers of international bodies.-ilep flash 5 (1991) 3
New course on health education/promotion. Health education promotion is given a high priority on the primary health care (PHC) agenda. There is a growing need for health workers to improve their health education/promotion skills. In response to this need a new 3-month certificate competency-based course in health promotion is being developed by the Education Resource Group for Health Systems of the Liverpool School of Tropical Medicine. The course is for people such as health, education, environmental, agriculture and community workers, who already have some experience of working in health education/promotion. The course aims to improve the ability of workers to design, plan, manage, implement and evaluate health education/promotion programs in the context of PHC. Methods of teaching will include groupwork, practical workshops and individual project work.
The first course will be held in January 1992 and annually thereafter. For more information write to: The Course Organizer (Health Education/Promotion for PHC), Department of International Community Health, Liverpool School of Tropical Medicine, Pembroke Place L3 5QA, U.K. Tel: 051 708 9393; Telex: 0627095 UNILPL G; Fax: 051 708 8733.-Leprosy Review 62 (1991) 231
Ofloxacin trials. ILEP Members have been asked to nominate field projects to participate in the forthcoming WHO/TDR/THELEP trials on new regimens containing ofloxacin. The new regimens will be tested for the treatment of multibacillary (MB) and paucibacillary (PB) leprosy. The request was made during an Interface Meeting held in Berne, June 1991, between ILEP Members and the ILEP Medical Commission. Prof. Grosset, Chair of the Medical Commission's research discipline, outlined the requirements for field participation.
For the MB trial each center should be able to recruit at least 116 untreated MB patients within 2 years. For the PB trial each center should be able to recruit at least 200 untreated patients within 2 years. Other requirements for both trials: 1. The control program should have been in existence for 5 years, with experience in implementing WHO MDT. 2. Patients should not have been previously treated with rifampin or ofloxacin. 3. There must be close supervision of treatment. 4. Each patient must be followed-up every 6 months for at least 5 years (for PB) or 8 years (for MB) after entry. 5. Any suspicion of relapse has to be closely monitored.
The full requirements are given in the protocols which have been issued to all ILEP Members with the report of the Interface Meeting. Nominations from ILEP Members should be sent to the Coordinating Bureau. Any field project interested in participating should contact their ILEP co-ordinator for further information. -ilep flash 5 (1991) 2
U.S.A. Canille Seminar Dates 1992-1993. The following seminars have been scheduled to be held at the GWL Hansen's Disease Center, Carville, Louisiana:
International Seminar on HP
September 13-17, 1992
September 13-17, 1993
Medical Seminar on HP
May 26-27, 1992
November 9-10, 1992
May 24-25, 1993
November 8-9, 1993
Management of Insensitive Feet
October 28-30, 1992
January 27-29,1993
October 27-29, 1993
Management of The Insensitive Hand
May 4-6, 1992
May 3-5, 1993
For further information, please contact: Dr. John R. Trautman, Acting Chief, Training and Education Branch, GWL Hansen's Disease Center, Carville, Louisiana 70721, U.S.A.
INMED's health education materials (EDTEC) program. The JOURNAL has received an updated Health Education Materials Catalog from the EDTEC program of the International Medical Services for Health (INMED). INMED offers these education materials of exceptional quality at low cost, in support of their common goal of improving world health. Included in this catalog are books, videos, posters and audio cassettes on such varied topics as breastfeeding, intestinal parasites, AIDS, and leprosy. Some of these materials are appropriate for the community, while others are most useful to trainers, planners, or doctors. All have in common a proven value as resources in the promotion of effective health practices in the developing world.
INMED is currently working with many NGOs and PVOs in 90 countries in Asia, Africa, and Latin America to provide them with quality medicines, medical supplies and, through the EDTEC program, health education materials. The EDTEC program collects, translates, and develops regionally appropriate health education materials in partnership with health agencies, communities, service clubs, companies, and the World Health Organization. EDTEC also forms model partnerships with selected agencies to share technical expertise through the creation of new health education materials. These materials are designed to meet needs identified by participating NGOs and PVOs. Ultimately, EDTEC seeks to promote knowledgeable and effective preventive health practices at the community level, both overseas and within the United States. For further information, contact: Martha Ewing, Health Education Coordinator, INMED, 45449 Severn Way, Suite 161, Sterling, Virginia 22170, U.S.A. - Materials from INMED
The Heiser Program for Research in Leprosy and Tuberculosis, 1992. In accordance with Dr. Victor George Heiser's stipulation at the time that he set up his fund in The New York Community Trust, the income is used not for treatment of patients but for basic laboratory research directed at a better understanding of the diseases and their bacterial agents. The ultimate aim is to find measures for the prevention and cure of these diseases that will serve to bring them under control. Two types of awards have been established to foster these objectives: (1) postdoctoral fellowships, designed to attract qualified and highly motivated young biomedical scientists to train in the relevant fields of research; and (2) small research grants that will support the training efforts of laboratories involved in research on leprosy and/or tuberculosis, or that will provide funds for the initiation of new research projects in the field.
The Heiser Program for Research in Leprosy, initiated at The New York Community Trust in 1974, has awarded over 125 postdoctoral fellowships and research grants over the past 17 years. The Program's scope has now been extended to include research in tuberculosis. A number of factors influenced this decision.
Tuberculosis, long a major infectious disease in the developing world, causing three million deaths each year, is now sharply on the rise in the industrial nations. Furthermore, much of this disease is being caused by bacteria that are resistant to the commonly used antibiotics. It is now clear that the bacterial agents, Mycobacterium leprae and M. tuberculosis, are closely related and have similar antigenic components. Thus, the search for effective means of immunization may well follow a common path for the two diseases. In light of these developments, a number of laboratories concerned with leprosy research are concurrently engaged in work on tuberculosis, and it seems logical to foster this combined attack.
The Heiser Program will thus continue its support of leprosy research, and at the same time will accept applications for the support of research on tuberculosis.
Postdoctoral Research Fellowships. To support young biomedical scientists in beginning postdoctoral training for research in leprosy and/or tuberculosis. Deadline for application: February 1.
Eligibility: Applicants should have an M.D., Ph.D., or equivalent degree. Although there is no age limit, candidates should be at an early stage of postdoctoral research training. There are no citizenship requirements. Generally, postdoctoral training should be planned in an institution other than that in which the applicant obtained his or her doctorate. Candidates should be interested in obtaining research training directly related to the study of leprosy and/or tuberculosis.
Terms of fellowships: Awards will be announced by May 1, and successful applicants must activate the fellowship between July 1 and December 31. Initial awards are for one year, renewable for a second year, at stipend levels between $20,000 and $25,000 per annum, adjusted according to such factors as number of dependents, previous postdoctoral experience, and cost of living in the training location. Economy air fare to the training location will be paid for the fellow and his or her family. A training allowance of $2000 will be provided annually to the training laboratory, and a maximum of $ 1500 per year will be paid toward health insurance. Successful applicants for these fellowships will be paid through the host institutions.
Instructions for making application: The applicant should submit, in English, one original and four copies of the following:
1. Face sheet-form provided.
2. Supplement No. 1-form provided.
3. Supplement No. 2-form provided.
4. Research proposal. The presentation should be a detailed description of the proposed research, not to exceed five single-spaced typewritten pages. Literature may be cited separately, and figures and tables may also be added.
5. Specific plans for the application of knowledge and experience gained through fellowship training and expected future in the field of research in leprosy and/or tuberculosis.
6. Curriculum vitae.
Additional items required:
7. Letter from proposed supervisor, indicating acceptance in the laboratory if fellowship is awarded.
8. Letters from three former teachers or supervisors as listed on the face sheet, to be forwarded directly to The Heiser Program, 450 East 63rd Street, New York, NY 10021.
Research Grants. To provide limited support to laboratories involved in research training on leprosy and/or tuberculosis, or to fund the initiation of new research projects. Deadline for application: February 1.
Eligibility: Applicants should be investigators who are experienced in research on leprosy and/or tuberculosis. Proposals should be of high scientific caliber and clearly related to these mycobacterial diseases. Startup funds may be requested for new projects that show promise of receiving support from other sources after preliminary results are obtained.
Terms of awards: Grants are limited in duration to one year. Decisions will be announced by May 1, and successful applicants must activate the grant between July 1 and December 31 of that year. Awards for research grants will not exceed $20,000, no more than 20% of which may be used for institutional overhead. These grants will not be awarded for clinical trials, and they may not be usedfor the salaries of personnel. Grants will be paid through the institution's fiscal office.
Instructions for making application: A face sheet is provided for the basic data and a summary of the proposed research project.
Additional items to be submitted:
1. A detailed description of the proposed project, not to exceed five single-spaced typewritten pages, exclusive of bibliography, tables, and figures.
2. Proposed budget.
3. Curriculum vitae and relevant bibliographies of scientists participating in the project.
The application must be in English, and one original and four copies should be submitted.
For further details and forms, contact: Mrs. Barbara M. Hugonnet, Director, Heiser Program for Research in Leprosy and Tuberculosis, 450 East 63rd Street, New York, New York 10021 U.S.A.
We were deeply saddened to learn of the death of Dr. R. H. Thangaraj on 30 July 1991. Dr. Thangaraj was Secretary of the International Leprosy Association from 1984-1988, and well known to those in the field of leprosy. A suitable obituary for Dr. Thangaraj will be forthcoming in the JOURNAL.