- Volume 58 , Number 3
- Page: 469–79
Studies of human leprosy lesions in situ using suction-induced blisters. 2. Cell changes and soluble interleukin 2 receptor (Tac peptide) in reversal reactions
ABSTRACT
To examine the pathogenesis of type 1 (reversal) reactions in leprosy, we studied cellular and soluble immunologic components of skin lesions in 10 patients with reactions, 24 active patients without reactions, and 33 control patients whose leprosy had been treated and cured. Cells and Tac-peptide levels were obtained f rom fluid aspirated f rom blisters induced by suction directly over representative skin lesions. During reversal reactions: a) the lesions contained an increased number and percentage of CD4+ (T-helper) cells; b) Tac-peptide levels were elevated in half of the lesions; c) the increases in Tac peptide and CD4+ cells were directly correlated; and d) systemic administration of corticosteroids appeared to cause a reduction in the intrale-sional CD4+ cell population. These findings were localized to the skin, and do not represent simple filtration of these components f rom the peripheral blood. We conclude that spontaneous lymphocyte activation in situ, primarily of CD4+ cells, is an important feature of reversal reactions, and may be an intermittent or cyclic phenomenon during the reaction. Findings in active patients without reactions are consistent with the hypothesis that differing states of immunologic equilibrium have been established in different portions of the leprosy spectrum. In reversal reactions we may, therefore, be examining immunologic processes set in motion when a pre-existing equilibrium has been upset by spontaneous, natural events. The mechanism of such spontaneous changes in immunity in leprosy is of considerable interest, not only to understand the reaction, but also to examine the underlying déterminants of delayed-type hypersensitivity and cell-mediated immunity in leprosy and the potential for artificially manipulating thèse responses, as proposed with vaccines or immunotherapy.RÉSUMÉ
Afin d'étudier la pathogénèse des réactions de type 1 (réverse) dans la lèpre, nous avons étudié les composants immunologiques cellulaires et solubles des lésions cutanées de 10 patients présentant des réactions, de 24 patients actifs sans réactions, et de 33 témoins dont la lèpre avait été traitée et guérie. Les cellules et les taux de peptide Tac furent obtenus à partir d'ampoules produites par aspiration directement au-dessus de lésions cutanées caractéristiques. Durant les réactions réverses: a) les lésions présentaient une augmentation du nombre et du pourcentage de cellules CD4 + (T-helper); b) les taux de peptide Tac étaient élevés dans la moitié des lésions; c) les augmentations des peptides Tac et des CD4 étaient directement córreles; et d) l'administration de corticostéroïdes par voie systémique apparut résulter en une réduction de la population des cellules CD4+ à l'intérieur des lésions. Ces observations étaient bien localisées au niveau de la peau, et ne sont pas dues à une simple infiltration de ces éléments à partir du sang périphérique. Nous concluons qu'une activation lymphocytaire spontanée in situ principalement de cellules CD4 + , est une caractéristique importante des réactions réverses, et peut être un phénomène intermittent ou cyclique durant la réaction. Les observations faites chez des patients actifs ne présentant pas de réactions sont compatibles avec l'hypothèse que différents états d'équilibre immuno-logique se retrouvent à différents niveaux du spectre de la lèpre. Au cours des réactions réverses, nous pouvons dès lors examiner des processus immunologiques déclenchés lorsqu'un déséquilibre pré-existant a été dérangé par des événements spontanés, naturels. Le mécanisme de tels changements spontanés dans l'immunité de la lèpre est d'un intérêt considérable, non seulement pour comprendre la réaction, mais aussi pour étudier les déterminants sous-jacents de l'hypersensibilité retardée et de l'immunité cellulaire dans la lèpre, et la possibilité de manipuler artificiellement ces réponses, comme on se propose de le faire avec des vaccins ou l'immunothérapie.RESUMEN
Para examinar la patogénesis de las reacciones del tipo 1 (reversas) en la lepra, estudiamos los componentes inmunológicos celulares y humorales en las lesiones dérmicas de 10 pacientes con reacción, en 24 pacientes activos sin reacción, y en 33 pacientes control cuya lepra había sido tratada y curada. Los niveles de células y del péptido Tac se midieron en el liquido de ampollas inducidas por succión directa sobre lesiones dérmicas representativas. Durante las reacciones reversas: a) las lesiones contuvieron un número aumentado de células CD4+ (T-cooperadoras), b) los niveles del péptido Tac estuvieron elevados en la mitad de las lesiones, c) los incrementos en los niveles del péptido Tac y de las células CD4+ mostraron una correlación directa, y d) la administración sistémica de corticoes-teroides causó una reducción en la población de células CD4+ intralesionales. Estos hallazgos estuvieron localizados en la piel y no representan una simple filtración de estos componentes a partir de sangre periférica. Concluimos que la activación espontánea de los lin-focitos in situ, fundamentalmente células CD4 + , es una característica importante de las reacciones reversas y puede ser un fenómeno intermitente o cíclico durante la reacción. Los hallazgos en los pacientes activos sin reacción son consistentes con la hipótesis de que se han establecido diferentes estados de equilibrio inmuno-lógico en las diferentes zonas del espectro de la lepra. Por lo tanto, en las reacciones reversas podemos estar examinando procesos inmunológicos desencadenados cuando el equilibrio pre-existente se ha alterado por eventos naturales espontáneos. El mecanismo de tales cambios espontáneos en la inmunidad de la lepra es de considerable interés, no solo para entender la reacción, sino también para entender los mecanismos de la inmunidad celular y la hipersensibilidad retardada en la lepra, con miras a su posible manipulación por vacunación o inmunoterapia.In man, Mycobacterium leprae causes a chronic, generally indolent infection, usually of greatest interest due to the very broad spectrum of cellular immunity and delayed hypersensitivity which results (21,22). The prolonged clinically uneventful course of this infection appears to be paralleled by an uneventful immunologic course as well. However, approximately 40%-50% of patients experience one or more episodes of acute inflammatory activity with fever, malaise, and often with neuropathy, termed "reactions." Two major categories of such reactions are recognized clinically, "reversal reactions" (RR) and erythema nodosum leprosum (ENL)-Jopling's types 1 and 2, respectively (9). The etiology and pathogenesis of both of these reactions are poorly understood.
In the middle or borderline (BL, BB, BT) portion of the immunopathologic spectrum of leprosy, the cellular immunologic responsiveness to M. leprae varies from weak (BL) to moderately strong (BT), and appears clinically to be capable of spontaneously increasing or decreasing to a limited extent. Only patients in these groups, which account for 55%-60% of the patients in our study area (T. Smith, personal communication), are at risk for reversal reactions.
A reversal reaction is experienced as the slow, insidious onset of a systemic illness with neuritis, iritis, fever and malaise, accompanied by exacerbation of pre-existing skin lesions. These have generally been ascribed to spontaneous shifts in cell-mediated immunity (CMI) or delayed-type hypersensitivity (DTH) to M. leprae (1,5). Until recently, little immunologic evidence had been obtained to support this view.
Recent studies have indicated that in skin lesions with RR the percentage of T-helper cells and of cytotoxic cells may be increased (12,14) and that cells bearing the interleukin-2 receptor (IL-2R) may also be increased in the epidermis (23). Systemically, elevated levels of soluble IL-2R (Tac peptide) have also been observed in the serum of patients with RR (28).
In view of evidence of both systemic and localized immunologic activation in reversal reactions, we are interested in delineating the mechanisms operating at the level of target tissues by simultaneously examining both mononuclear cell subsets and soluble indicators of immunologic activation in the skin lesions. We asked whether the presence of different lymphocyte subsets could be correlated with the level of soluble IL-2R (Tac peptide) in leprosy lesions in vivo, and whether localized immunologic activity in the skin was demonstrably greater than in the peripheral blood.
To examine these questions, we measured the numbers of T-helper(CD4 +) cells and Tac peptide in the fluid of blisters induced directly over RR lesions under controlled conditions, comparing them to the results obtained with similar blisters in patients with active leprosy without reactions and in cured, inactive patients. The Tac-peptide results in blisters were also compared to those in matched sera, where possible.
MATERIALS AND METHODS
Patients. Ten patients with RR (7 BL, 3 BB), 24 active patients without reaction, and 33 cured, inactive patients were recruited from among the inpatients at McKean Rehabilitation Institute, Chiang Mai, Thailand. The latter two groups have been described in the first part of this series (17). A representative lesion was biopsied in each of the patients with active disease, and the patients were classified by clinical and pathologic criteria according to the five-part scale of Ridley and Jopling (18). Only 2 of the 10 patients with RR were female; the age distribution was also similar to that of patients without RR (median age 47 years, range 19-61).
Careful clinical histories and physical examinations were performed for evaluation and daily follow up of the reactions. The time of onset and prior duration of the reaction were determined as clearly as possible, and studies commenced within 24 hr of admission. When treatment with corticosteroids was initiated before or during the study, the precise timing of medication was recorded with respect to the time of collection of each laboratory specimen.
Blister induction. After obtaining informed consent from each volunteer, four nearly identical blisters were induced by gentle continuous suction, as previously described (17), directly over a representative skin lesion, or in inactive patients, in clinically healthy skin. No skin-test antigen or other material was injected. The fluid-filled blisters were covered by an inverted plastic cap to prevent breakage, and each was aspirated only once. To examine the reproducibility of the results from blister fluids, paired blisters were aspirated from several of the inactive patients, each aspirate being handled and assayed individually.
Handling of blister fluids. Each blister was sampled only once, at 24, 30, 48, 72, or 96 hr after induction. (In reproducibility studies with paired blisters, some samples were taken at 6, 12, and 18 hr.) Fluid was aspirated into a heparinized 1.0-cc tuberculin syringe, and after measuring the volume in the syringe, it was washed through a 13-mm, 0.22-μm polyvinylidine difluo-ride filter ("Durapore"; Millipore Corp., Bedford, Massachusetts, U.S.A.). The cell-free material was flushed through the filter with RPMI 1640 tissue culture medium, and the first 1.0 cc of filtrate was divided into aliquots and frozen at - 70ºC until assayed.
Cell staining and counting. The indirect immunoperoxidase method described earlier (18) was employed, using monoclonal antibodies Leu 4, Leu 3 or T4, and Leu 2 or T8 to identify subpopulations of lymphocytes bearing CD3, CD4, and CD8 receptors, respectively.
Assay for Tac peptide. Tac-peptide levels were determined using an ELISA employing monoclonal antibodies anti-Tac and 7G7/ B6, recognizing different epitopes of IL-2R, as previously described (19). Briefly, 100-μl aliquots of three dilutions of each blister fluid were placed in wells of microliter plates coated with anti-Tac. These were overlaid with a 4/1000 dilution of FITC-conjugated 7G7/B6, followed by alkaline phosphatase-conjugated rabbit anti-FITC, and by the substrate, p-nitrophenyl phosphate (1 mg/ ml; Sigma Chemical Co., St. Louis, Missouri, U.S.A.), with thorough washing between each step. Absorbance was determined at 405 nm in an automated 96-well ELISA reader. Serial dilutions of a standardized IL-2-containing supernatant were used to define a reference curve, for which the undiluted supernatant was assigned a maximum value of 1000 units/ml. The units (U) of Tac peptide in the test samples were calculated from this reference curve.
Radioiodination of protein. To determine the optimal recovery of protein in this filter system, we first examined aliquots of normal human serum which had been labeled with 125I using Iodobeads (Pierce Chemical Co., Rockford, Illinois, U.S.A.) (11). After centrifugation to remove the beads, 0.1 -cc aliquots of labeled serum were aspirated into 1.0-cc tuberculin syringes, diluted to 0.4 cc with medium, and filtered exactly as described above.
Total protein measurements. The Coomassie blue method (6) was used to measure total protein in blister fluids and matched serum samples. All results are expressed as mg protein per ml fluid; for each blister this was calculated by multiplying the test result by the dilution factor for that sample.
Calculation of a Tac peptide/protein index. To differentiate the local production of Tac peptide in the skin from Tac which entered a blister by filtration from the plasma, we calculated a "Blister Tac Index" modeled after the cerebrospinal fluid immunoglobulin index, widely used to identify local production of IgG in cerebrospinal fluid (6). From Tac peptide (U/ml) and total protein (mg/ml) concentrations in blister fluids and matched sera, the blister Tac/protein ratio was divided by the serum Tac/protein ratio, as follows:
Blister Tac Index
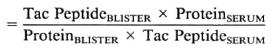
Statistical analysis. The nonparametric Wilcoxon test was used to compare ranked data from different groups of patients (25). Correlation coefficients were calculated according to the method of Pearson (26).
RESULTS
Lymphocyte subsets in lesions with RR. All patients studied during RR in this series were classified BB or BL, and the results have therefore been compared to lesions in 11 BB and BL patients without RR (18). The T-helper: suppressor-cytotoxic ratio (CD4+ : 3CD8 +) was higher in lesions with RR than in lesions without RR, median values being 3.0 and 1.0, respectively (p = 0.01) (Fig. 1). This difference was even greater when only BL lesions were compared (p < 0.001). The absolute number of CD4+ cells per ml was also greater in BL lesions with RR than in quiescent BL lesions (p < 0.001) (Fig. 2), but this difference was not observed when BB lesions were included. Wide individual variation in absolute cell numbers was observed in blisters over different RR lesions, as was noted earlier in lesions of all classifications without reaction (17), and is most probably due to individual variations in the depth and quantity of the dermal infiltrate.
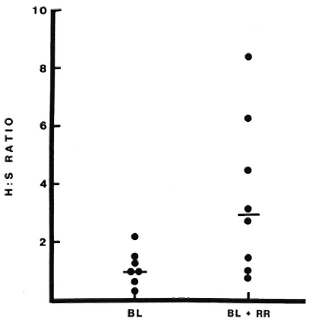
Fig. 1. CD4 + : CD8 + (H : S) ratios in lesions with or without reversal reactions (RR). Lymphocytes were collected on filters from blisters induced over lesions with or without RR and stained immunocytochemi-cally as described. None of the reactions had been treated with corticosteroids at the time of sampling. Each point represents results from one blister; bars indicate median values.
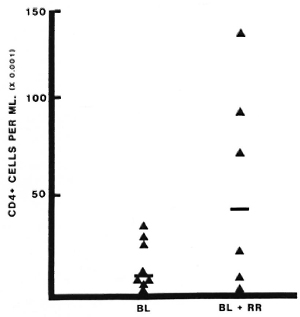
Fig. 2. Absolute numbers of CD4+ cells in lesions with or without reversal reactions (RR). Lymphocytes were collected and stained as described (Fig, 1 and text). Number of CD4+ cells per ml was calculated from the total number counted and the volume of blister fluid recovered. Each point represents one blister; bars indicate median values.
Optimal collection of protein in filtrate. The first 1.0 cc of filtrate contained 86% of the total 125I in the original 0.1-cc sample, after which recoverable protein fell to a low, plateau level (data not shown). A final volume of 1.0 cc (in some studies, 1.5 cc) was therefore used for all studies of blister fluids.
Reproducibility of results with blister fluids. In 53 paired samples from 31 inactive patients, median Tac-peptide levels varied from 400-800 U/ml at times from 24-96 hr, ranging from 200-2600 U/ml (Fig. 3). The correlation coefficient for these paired samples was 0.58 (p < 0.01). No correlation was noted between the cutaneous Tac-peptide levels in these patients and the immunopathologic classification of their disease when it had been active. When blister volumes were less than 50 μd, values of the Tac peptide tended to be greater; the statistical association between these low volumes and higher values of Tac peptide was significant but weak (p = 0.0003; r = 0.345) and disappeared when volumes were 50 μl or more. In subsequent analysis of the results from active patients, with or without reversal reaction, exclusion of data from small blisters (< 50 μl) did not affect the results. Variation was not related to the duration of the blister before aspiration, but may be partially due to local differences in trauma to the dermis during blister induction, reflected, for example, in the presence of red blood cells or hemoglobin in the fluid. These were not systematically measured in this study.
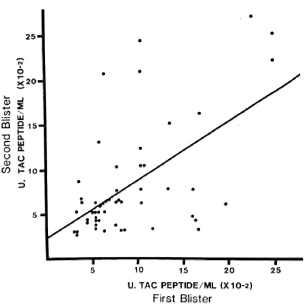
Fig. 3. Reproducibility of Tac-peptide measurements in paired blisters. Four blisters were induced by suction on healthy skin of cured, inactive leprosy patients. Fluid was aspirated from blisters at different times in pairs, and each aspirate was assayed separately. Each point represents results from one pair of blisters. No correlation was observed between Tac-peptide levels and the immunopathologic type of leprosy from which the patient had recovered.
Cutaneous Tac-peptide levels in active and inactive leprosy. In blisters induced on the skin of cured, inactive patients, median levels of Tac peptide varied from 400-800 U/ml (Fig. 4). No significant difference was observed between those individuals who had recovered from different types of leprosy.
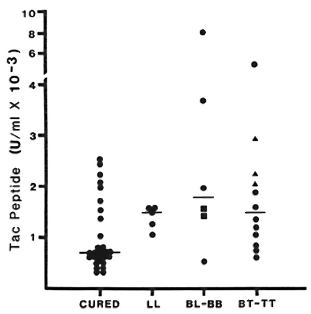
Fig. 4. Cutaneous Tac-peptide levels in skin blisters from cured, inactive patients and in active lesions without reaction. Results shown arc from samples aspirated 48 hr after blister induction. "Cured" ( ) refers to fully treated, inactive patients; other patients are grouped by Ridley-Jopling classification: BB =
) refers to fully treated, inactive patients; other patients are grouped by Ridley-Jopling classification: BB =  ; TT = ▲; each point represents Tac-peptide level measured in one blister; bars indicate median levels.
; TT = ▲; each point represents Tac-peptide level measured in one blister; bars indicate median levels.
In blisters induced directly over active lesions (without any reaction), median Tac-peptide levels varied between 1100 U/ml and 1600 U/ml at all times studied and for all types of leprosy (Fig. 4). No statistically significant difference was observed between immunopathologic types of leprosy at any time studied. Tac-peptide levels remained relatively constant over the study period in most patients. When elevations were observed, they tended to occur 48-72 hr after blister induction. The range of values for Tac peptide was greater among blisters over active lesions, primarily reflecting higher values in a small number of BT patients.
Cutaneous Tac-peptide levels in lesions in RR. In 6 of 13 episodes of RR in BB or BL patients, the levels of Tac-peptide in blisters over the reacting lesions were higher (median 7000-8000 U/ml) than those seen in uncomplicated lesions (Fig. 5). The tendency for peak levels of Tac peptide to occur at 48 or 72 hr after induction was more prominent in some of these RR lesions (Fig. 5). Cutaneous fluid from the other seven episodes of RR contained low levels of Tac peptide, in the range seen for uncomplicated BL-BB leprosy. No correlation has been found between the low or high levels of Tac peptide and the patients' age, sex, classification, treatment or duration of active leprosy.
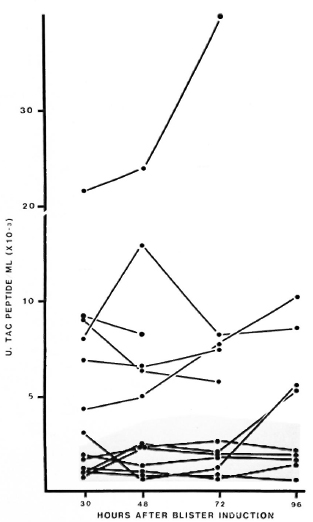
Fig. 5. Cutaneous Tac-peptide levels in lesions with reversal reactions. Each point represents results from one blister; points are connected to show sequential results from each patient. Shaded area = range of results from active nonreacting BL-BB lesions.
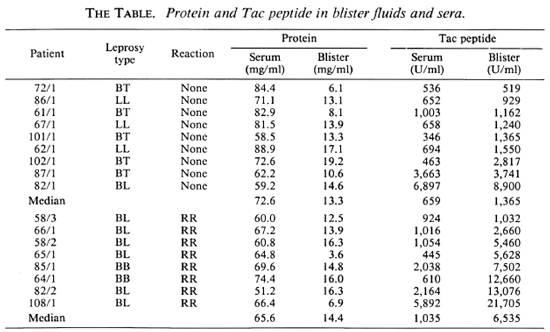
Correlation of serum and blister Tac-peptide levels. A comparison of Tac-peptide levels in blisters and in serum drawn simultaneously is presented in The Table. In lesions without reactions, the median Tac level in blisters was twice that of the sera, while in lesions with RR the median level in blisters was six times greater than in sera. In contrast, the median ratio of total protein in blisters/serum was similar in nonreacting and RR lesions (median = 18% and 22%, respectively).
The total protein was therefore used as an estimator of passive filtration of protein into blisters by calculating a "Blister Tac Index" as described. The median Index for RR lesions was 19.2, compared to 11.6 for nonreacting lesions (Fig. 6). Seven of eight RR lesions had an Index higher than 12, the highest seen in this small number of non-reacting BB/LL lesions, while 3 of 8 had an Index higher than 23, the highest seen in nonreacting BT lesions.
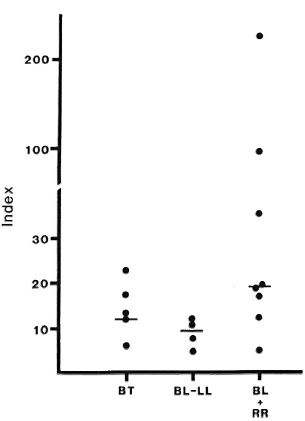
Fig. 6. Blister/Serum Tac Indices: patients with or without reversal reaction (RR). Total protein concentrations and Tac-peptide levels were determined in matched samples of blister fluid and scrum from patients with reversal reaction, or with leprosy without reaction. The Blister Tac Index was calculated as described. Bars indicate median indices for each group; median Index for lesions in reaction is significantly greater than for lesions without reaction (p < 0.025).
Correlation of Tac peptide with CD4 + cells and effect of corticosteroids. The analyses of Tac-peptide levels and numbers of CD4+ lymphocytes were performed on 15 blisters on RR lesions in five patients studied prior to corticosteroid treatment. A direct correlation was observed between increasing Tac-peptide levels and increasing numbers of CD4+ cells (r = 0.82, p < 0.01). This is illustrated in Figure 7A, in'which CD4 + cell counts are presented for samples grouped according to low or high Tac-peptide levels (< 3000 U/ml or > 5000 U/ml, respectively). In nine blisters with low Tac-peptide levels, a median of 17,640 CD4+ cells/ml was found, compared to a median of 116,400 CD4 + cells/ml in six blisters with high Tac-peptide levels.
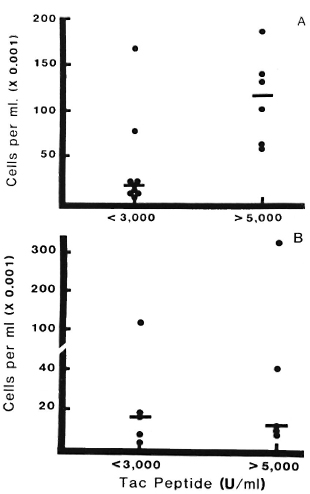
Fig. 7. CD4+ lymphocytes and Tac-peptide levels in reversal reactions with or without corticosteroid treatment. Each point represents results from one blister; bars indicate median numbers of CD4+ cells in each group. Data are grouped into those with low Tac levels (< 3000 U/ml) or high levels (> 5000 U/ml).
A = Reacting lesions prior to corticosteroid treatment. Significantly greater numbers of CD4+ cells were found in samples with high Tac-peptide levels (p < 0.01).
B = Reacting lesions in patients after corticosteroid treatment. Median number of CD4+ cells was low even when Tac-peptide levels exceeded 5000 U/ml; there was no significant difference in CD4+ cell counts between groups with low or high Tac-peptide levels (p >0.1).
Preliminary data concerning the effect of corticosteroid treatment on these parameters in RR lesions are presented in Figure 7B. During the 96-hr period examined with this blister technique, treatment with corticosteroids did not appreciably alter the proportion of high versus low Tac-peptide samples -five blisters had high levels and five had low levels, similar to the proportion in untreated RR lesions (six high and nine low). In contrast, the number of CD4+ cells was affected by prednisone treatment. In lesions with high Tac-peptide levels, a median of only 12,090 CD4+ cells/ml was observed in prednisone-treated RR lesions compared to 116,400 CD4+ cells/ml in the untreated RR lesions.
DISCUSSION
This study has attempted to evaluate lymphocyte function in situ in leprosy skin lesions by correlating changes in T-cell subsets with Tac-peptide levels, a reflection of T-cell activation. The findings indicate that a locally generated, cell-mediated immunologic reaction is present in the acutely inflamed skin lesions of reversal reactions (RR) in this disease. Increases in the CD4 + (helper) phenotypes described in early studics of RR lesions (12,14) could not be taken to imply the functional state of those cells. Similarly, the demonstration of elevated levels of circulating Tac peptide in patients with RR (28) did not reveal whether skin lesions were primary sites of immunologic activity or only secondary passive sites for the reaction of various immunologic components, including those circulating systemically.
All of the type 1 reactions reported here were "upgrading" reactions, primarily in BL patients, and the results indicate that RR are manifestations of a spontaneous increase in DTH (and possibly CMI) in leprosy patients. Thus, a) an increase in the CD4+ lymphocyte population was observed in BL lesions in RR; b) intracutaneous levels of Tac peptide were elevated in a subset of these lesions; c) the increases in CD4 + cells and Tac peptide were directly correlated; and d) systemic administration of corticosteroids caused a reduction in the number of CD4+ cells in the lesions.
The upward shift in the percentage of CD4+ cells in lesions was due to an absolute increase in the number of these cells. This agrees with previous biopsy results in RR (4,12,l4) and with increases of this cell type seen in DTH responses to tuberculin (PPD) in biopsies (16) and in blisters (10). The BB lesions with reaction did not show this trend, and preliminary results suggest that such a shift is not seen in BT lesions with this reaction, either (Scollard and Bhoopat, unpublished results). Lesions of BB and BT leprosy may have enough of these cells already present to perform the required functions, while RR in BL lesions requires an increase in CD4+ cells. Cooper and colleagues (4) have reported that the T-"helper/memory" subset (2H4-UCHL1 +) of CD4+ cells predominates in RR lesions, as opposed to nonreacting lepromatous lesions. With the methods employed, we were unable to perform the multiple-labeling techniques required to further discriminate between subsets of CD4+ cells, but such studies are now in progress.
Models of CMI in vitro indicate that the production and expression of IL-2R are essential steps in the initiation and maintenance of CMI (3). The expression of IL-2R in cell membranes in vitro is accompanied by the release into the culture supernatants of soluble Tac peptide (20); CD4+ cells are the major, but not only, source of Tac peptide (15). Increased circulating levels of Tac peptide have been observed in serum from patients with lymphoreticular malignancies (29) and inflammatory conditions (27), including RR in leprosy (28).
The Tac-peptide levels of 5000-39,000 U/ml observed in half of the RR lesions are much greater than the maximum level of 2800 U/ml previously observed in response to 1.25 tuberculin units (TU) of PPD in-tradermally (24), and therefore indicate a substantial degree of lymphocyte activation. The observation that the total protein filtered into blisters from nonreacting and reacting lesions is very similar, while blister Tac-peptide levels in RR arc much higher than circulating levels, provides strong evidence of the local, cutaneous origin of the Tac peptide found in blisters. These results are also in agreement with the increased synthesis of mRNA, IP-10 protein, and gamma-interferon in RR lesions (4).
We found elevated Tac-peptide levels in blisters and in serum in only half of the patients with RR in this study, while high circulating levels were observed in all RR patients in an earlier study (28). In part, this may be due to differences in the criteria and manner of selection of patients; the present study recruited patients sooner after admission and clinically is probably a more diverse sample, including more patients with less severe reactions. Variations in Tac-peptide levels could result if different mechanisms produced the same clinical syndrome of RR, but it seems more likely that the variation was seen because RR lesions in different patients were sampled at different points during the course of the reaction. Because the onset of this clinical syndrome is usually insidious, retrospective assessment of the time of onset of the reaction is likely to be quite inaccurate. However, no better method of "dating" these reactions is available. Since immunologic events within lesions are dynamic, different results would probably be obtained if the lesion were sampled during the onset, peak, or resolving phases of the reaction, for example.
Moreover, Tac-peptide release and cellular expression of IL-2R are known to be brief, transient events in vitro, dependent upon the continued presence of antigen and adequate levels of IL-2 (2). We have also observed this in vivo: after a single intradermal challenge with a low dose of PPD, the release of Tac peptide in human volunteers peaked at 48-72 hr and then declined (2). Although the antigens responsible for initiating RR have not been identified, it is widely assumed that they are M. leprae antigens (8). Since the addition of antigen to cells can restimulate IL-2R expression (7), it is possible that the M. leprae antigens involved in RR are expressed or released irregularly, precipitating the transient, intermittent expression and release of IL-2R during the course of the reaction.
In lesions of RR patients treated with corticosteroids, the decreased number of CD4 + cells corresponds to the clinical response of these reactions to corticosteroids, but contrasts with the more prolonged elevation of Tac peptide in some lesions. This is probably residual Tac peptide, "trapped" in the blisters, although we cannot exclude the possibility that there is continued local (intracutaneous) Tac peptide released from cells for a few days after the initiation of corticosteroids. In an earlier study, a consistent decline in circulating Tac peptide was observed in all RR patients after corticosteroid treatment, over a period of several weeks (28).
The most dynamic changes in the patient's immune response to M. leprae have probably taken place by the time immunologic studies are usually done, since the processes which determine an individual patient's final position in the leprosy spectrum probably occur in the early period after infection, long before the disease becomes clinically evident. In typical, established, nonreacting leprosy lesions, the number and percentage of CD4+ cells in skin lesions have been observed to increase progressively from the lepromatous to the tuberculoid poles of the spectrum (13), as noted also in the first report in this series (17). The present data, however, reveal similar Tac-peptide levels in nonreacting lesions from all portions of the spectrum. Together, these findings suggest that an equilibrium or "steady state" has been established in which different types of non-reacting leprosy lesions have accumulated differing numbers of T-helper cells, but nevertheless show a low, similar level of immunologic activation.
The direct correlation of the increased numbers of T-helper cells and elevated Tac peptide in some RR lesions appears to represent a disruption of this equilibrium in RR, employing immunologic processes set in motion by natural, spontaneous events. If so, then some of the immunologic changes in these reactions might involve an attempt to re-establish equilibrium, and in doing so they might recapitulate some of the immunologic events involved in the development of CMI during the early silent period of infection when the patient's immunologic responsiveness to M. leprae is being established for the first time. The mechanism of such spontaneous change in immunity in leprosy is of considerable interest, not only to understand the reaction but also to examine the underlying determinants of DTH and CMI in leprosy and the potential for artificially manipulating these responses, as proposed with vaccines or immunotherapy.
Acknowledgments. We are deeply indebted to Miss Atcha Masarat, R.N., for her excellent nursing assistance; to Utaiwan Utaipat and Rawiwan Kurapakorn for their skilled technical assistance; to Dr. Robert McGee for his generous statistical advice; and to Ms Marlene Bohle for assistance in preparing the manuscript. Portions of this work were carried out in the laboratories of the Research Institute for Health Sciences (RIHES), Chiang Mai University.
This work was supported by a grant from the Science and Technology Program of USAID, and by a grant to RIHES from the Tropical Diseases Research and Training Program of the World Health Organization.
REFERENCES
1. Bjune, G., Barnetson, R. St. C, Ridley, D. and Kronvall, G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of the lesions. Clin. Exp. Immunol. 25(1976)85-94.
2. Cantrell, D. A., and Smith, K. A. Transient expression of interlcukin-2 receptors; consequences for T cell growth. J. Exp. Med. 158(1983)1895-1911.
3. Cantrell, D. A. and Smith, K. A. The interleu-kin-2 T-cell system; a new cell growth model. Science 224(1984)1312-1317.
4. Cooper, C. L., Mueller, C, Sinchaisri, T. A., Pirmez, C, Chan, J., Kaplan, G., Young, S. M., Weissman, I. L., Bloom, B. R., Rea, T. H. and Modlin, R. L. Analysis of naturally occurring dclayed-type hypersensitivity reactions in leprosy by in situ hybridization. J. Exp. Med. 169(1989)1565-1581.
5. Godal, T., Samuel, D. R., Ross, W. F. and Lofgren, M. Mechanism of "reactions" in borderline tuberculoid (BT) leprosy. Acta Pathol. Microbiol. A Suppl. 236(1973)45-53.
6. Grant, G. H., Silverman, L. M. and Chris-tenson, R. H. Amino acids and proteins. In: Fundamentals of Clinical Chemistry. 3rd cd. Tietz, N. R., cd. Philadelphia: W. B. Saunders, 1987, pp. 339-341.
7. Greene, W. C and Leonard, W. J. The human interlcukin-2 receptor. Ann. Rev. Immunol. 4(1986)60-95.
8. Harboe, M. The immunology of leprosy. In: Leprosy. Hastings, R. C, cd. New York: Churchill Livingstone, 1985, p. 78.
9. Jopling, W. H. Leprosy reactions (reactional states). In: Handbook of Leprosy. London: William Heinemann Medical Books, 1971, p. 42.
10. Kenney, R. T., Rangdaeng, S. and Scollard, D. M. Skin blister immunocytology: a new method to quantify cellular kinetics in vivo. J. Immunol. Methods 97(1987)101-110.
11. Markwell, M. A. K. A new solid-state reagent to iodinatc proteins. 1. Conditions for the efficient labelling of antiserum. Anal. Biochcm. 125(1982)427-432.
12. Modlin, R. L., Gebhard, J. F., Taylor, C. R. and Rea, T. H. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin. Exp. Immunol. 53(1983)17-24.
13. Modlin, R. L., Hofman, R. M., Taylor, C. R. and Rea, T. H. T-lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 8(1983)182-189.
14. Narayanan, R. B., Laal, S., Sharma, A. K., Bhutani, L. K. and Nath, I. Differences in predominant T cell phenotypes and distribution pattern in reactional lesions of tuberculoid and lep-romatous leprosy. Clin. Exp. Immunol. 55(1984)623-628.
15. Nelson, D. L., Rubin, L. A., Kurman, C. C, Fritz, M. E. and Boutin, B. An analysis of the cellular requirements for the production of soluble inter-leukin-2 receptors in vitro. J. Clin. Immunol. 6(1986)114-120.
16. Platt, J. L., Grant, B. W., Eddy, A. A. and Michael, A. F. Immune cell populations in cutaneous delayed-type hypersensitivity. J. Exp. Med. 158(1983)1227-1242.
17. Rangdaeng, S., Scollard, D. M., Suriyanon, V., Smith, T., Thamprasert, K. and Thee-tranont, C. Studies of human leprosy lesions in situ using suction-induced blisters. 1. Cellular components of new, uncomplicated lesions. Int. J. Lepr. 57(1989)492-498.
18. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
19. Rubin, L. A., Kurman, C. C, Biddieson, W. E., Goldman, M. D. and Nelson, D. L. A monoclonal antibody, 7G7/B6, that binds to the human 1L-2 receptor at an epitope distinct from that recognized by IL-2 oranti-Tac. Hybridoma 4(1985)91-102.
20. Rubin, L. A., Kurman, C. C, Fritz, M. E., Biddieson, W. E., Boutin, B., Yarchoan, R. and Nelson, D. L. Soluble interIeukin-2 receptors are released from activated human lymphoid cells in vitro. J. Immunol. 135(1985)3172-3177.
21. Skinsnes, O. K. The immunological spectrum of leprosy. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Baltimore: Williams and Wilkins, 1964, pp. 156-182.
22. Sansonetti, P. and Lagrange, P. H. The immunology of leprosy: speculations on the leprosy spectrum. Rev. Infect. Dis. 3(1981)422-469 (274 refs).
23. Scollard, D. M., Rangdaeng, S., Suriyanon, V., Theetranont, C, Smith, T. and Chang, P. Increased epidermal I1-2R+ cells in leprosy reversal reactions. (Abstract 417) Lab. Invest. 56 (1987) 70A.
24. Scollard, D. M., Wagner, D. K. and Nelson, D. L. Release of soluble interleukin-2 receptors (Tac peptide) in vivo during human immune responses to tuberculin. Clin. Immunol. Immuno-pathol. 46(1988)450-455.
25. Sokal, R. R. and Rohlf, F. J. Biometry. San Francisco: W. H. Freeman & Co., 1969, p. 391.
26. Sokal, R. R. and Rohlf, F. J. Biometry. San Francisco: W. H. Freeman & Co., 1969, p. 498.
27. Tomkinson, B. E., Wagner, D. K., Nelson, D. L. and Sullivan, J. L. Activated lymphocytes during acute Epstein-Barr virus infection. J. Immunol. 139(1988)3802-3807.
28. Tung, K. S. K., Nelson, K. E., Rubin, L., Wagner, D., Umland, E., Schauf, V., Scollard, D., Vithayasai, P., Vithayasai, V., Worobec, S., Smith, T. and Suriyanon, V. Serum soluble in-terleukin-2 receptor levels in leprosy patients. Clin. Exp. Immunol. 69(1987)10-15.
29. Wagner, D. K., Kiwanuka, J., Edwards, B. K., Rubin, L. A., Nelson, D. L. and Magrath, I. T. Soluble interleukin-2 receptor levels in patients with undifferentiated and lymphoblastic lymphomas: correlation with survival. J. Clin. Oncol. 5(1987)1262-1274.
1. M.D., Ph.D., Department of Pathology, University of Hawaii School of Medicine, Leahi Hospital, 3675 Kilauca Avenue, Honolulu, Hawaii 96816, U.S.A.
2. M.D.; Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
3. Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
4. M.D.; Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
5. M.D., Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
6. M.D.; Immunophysiology Section, Metabolism Branch, National Cancer Institute, Bethesda, Maryland, U.S.A.
7. M.D., Immunophysiology Section, Metabolism Branch, National Cancer Institute, Bethesda, Maryland, U.S.A.
8. M.D., McKcan Rehabilitation Institute, Chiang Mai, Thailand.
Received for publication on 17 July 1989.
Accepted for publication in revised form on 19 April 1990.