- Volume 58 , Number 3
- Page: 480–90
Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses
ABSTRACT
The antibody responses of 100 previously untreated multibacillary (MB) leprosy patients to one protein and two carbohydrate antigens were examined: 94% of the patients had Mycobacterium leprae-specific antibodies; 89% directed to the species-specific epitope on phenolic glycolipid (PGL-I), 89% against the specific epitope on the 35-kDa protein, and 94% against one or both of the two. By contrast, 67% of the patients had anti-lipoarabinomannan (LAM) antibodies. There were trends for the seropositivity rate and the antibody level to rise with the increasing extent of the disease and as patients moved to the polar lepromatous end of the spectrum. The bacillary load, as measured by the bacterial index, was moderately correlated with the IgM anti-PGL-I and the anti-35-kDa antibody levels and, to a lesser extent, with the IgG antibodies directed at the common mycobacterial carbohydrate LAM. The sensitivity of the IgM anti-PGL-I antibodies for detecting smear-positive MB disease was 91%; that for the anti-35-kDa antibodies was 92%.RÉSUMÉ
Les réponses en anticorps pour deux hydrates de carbone ct pour une protéine antigéniques ont été étudices chez 100 malades atteints de lèpre multibacillaire (MB) et non traités. Des anticorps spécifiques pour Mycobacterium leprae ont été relevés chez 94% des malades; 89% présentaient des anticorps dirigés contre l'épitope, spécifique d'espèce, de l'antigène phénogly-colipidique (PGL-I), 89% révélaient des anticorps contre l'épitope spécifique situé sur la protéine 35-kDa, et 94% contre l'un et l'autre de ces deux epitopes. Par contre, 67% des malades révélaient la présence d'anticorps anti-lipoarabinomannan (LAM). On a relevé une tendance à une élévation du taux de séropositivité et des taux d'anticorps correspondant à l'étendue de la maladie, et ceci d'autant plus que le type clinique présenté par les malades se rapprochait du pôle lépromateux du spectre. La charge bacillaire, telle qu'elle a pu être mesurée par l'index bactériologique, présentait une certaine corrélation avec les taux d'anticorps IgM anti-PGL-I et anti-35-kDa, et dans une moindre mesure, avec les taux d'anticorps IgG dirigés contre l'hydrate de carbone LAM commun aux mycobactérics. La sensibilité des anticorps IgM anti-PGL-I pour détecter une lèpre multibacillaire à frottis positif était de 91%; cette sensibilité était de 92% pour les anticorps anti-35-kDa.RESUMEN
Se examinó la respuesta en anticuerpos de 100 pacientes con lepra multibaciiar (MB) en contra de un antígeno proteico y 2 carbohidratos del Mycobacterium leprae: 94% de los pacientes tuvieron anticuerpos específicos contra el M. leprae; 89% dirigidos contra el epitope especifico de la especie en el glicolípido fenólico 1 (PGL-1), 89% contra el epitope específico sobre la proteína de 35 kDa, y 94% contra uno de ellos o contra ambos. En contraste, el 67% de los pacientes tuvieron anticuerpos anti-lipoarabinomanana (LAM). La frecuencia de seropositividad y los niveles de anticuerpos tendieron a aumentar conforme aumentaba el grado de la enfermedad y conforme ésta se acercaba al extremo lepromatoso del espectro. La carga bacilar, medida por el índice bacteriano, correlacionó moderadamente con los niveles de anticuerpos IgM anti-PGL-ly anti-35 kDa, y en menor grado con los niveles de anticuerpos IgG anti-LAM. La sensibilidad de los anticuerpos IgM anti-PGL-1 para detectar la enfermedad MB fue del 91%, la sensibilidad de los anticuerpos anti-35 kDa fue del 92%.Recent developments in the serology of leprosy raise the question of the operational value of serological measurements in the diagnosis and management of the leprosy patient. Species-specific serological responses have been demonstrated to the capsular phenolic glycolipid (PGL-I) (3), and to a range of peptide epitopes specific to Mycobacterium leprae. These include the 35-kDa (23), 36-kDa (15), and the 18-kDa (6) proteins of M. leprae. In addition, common mycobacterial carbohydrate antigens, such as lipoarabinomannan (LAM) (17) and phosphatidyl inositol mannosidc (PIM) (Fournie, et al, 1990, unpublished), stimulate a strong B-cell response in leprosy patients. The antibody levels to both species-specific epitopes and common mycobacterial antigens arc higher in lepromatous patients and fall toward the tuberculoid pole of the spectrum. In general, the initial studies with these antigens have included mixed groups of treated and untreated patients (1,3,7,15-l9). Since anti-M. leprae antibody levels fall with therapy at a variable rate (1,5,22), the exact relation between disease activity and antibody level has not always been clear (17).
We have therefore studied the serological responses of 100 previously untreated multibacillary (MB) patients diagnosed under field conditions to examine the role of antibody measurement in this clinical setting. Our aims were: a) to examine the differential responses to one protein and two carbohydrate antigens; b) to compare the serological responses to the three antigens with clinical parameters in MB patients; and c) to determine whether antibody levels can supplement or even replace the bacterial index (BI) as a measure of bacterial load.
MATERIALS AND METHODS
Patients. The patient group consisted of 100 self-referred, previously untreated patients with midborderline (BB), borderline lepromatous (BL), or polar lepromatous (LL) leprosy diagnosed on clinical findings according to the Ridley-Jopling classification (20) in three leprosy clinics in Nepal. The clinical diagnosis was considered the major factor in assignment to chemotherapy, and this group is referred to as multibacillary (MB) despite the negative skin smears in 10 patients. Previous therapy was assessed by careful interview, and patients with any features or suspicion of past therapy were excluded. From data collected prospectively, the duration of disease was estimated by the length of the history before presentation and the cumulative disability index (DI) on a scale of 0 to 18, which was the sum of the changes in the four limbs and both eyes, each ranked on a scale of 0 to 3.
The clinical extent of disease was assessed by the number of skin patches, the presence of diffuse infiltration of the skin and/or nodules, and the number of involved nerves as evidenced by unequivocal enlargement of the nerve trunk or established nerve-function loss in the distribution of the nerve. The mean BI was calculated from slit-skin smears at four sites, including one clinical lesion, using the logarithmic scale from 1 +-6+ (20). Serum samples were collected and stored in 0.01% sodium azide at - 20ºC until testing. Control sera from healthy Nepali medical and nursing students were kindly provided by Mr. B. Pokherel, Institute of Medicine, Kathmandu.
Serological assays. IgM anti-phenolic glycolipid (PGL-I) antibodies were measured by an enzyme-linked immunosorbent assay (ELISA) using the glycoconjugate di-saccharide bovine serum albumin (dBSA, provided by the IMMLEP program of the World Health Organization) (3,12). Briefly, the wells of a Cooke microtiter tray (Dy-natech, Alexandria, Virginia, U.S.A.) were coated with 100 μl dBSA 250 ng/ml in 0.05 M sodium carbonate buffer, pH 9.6; blocked with 200 μl of 1.0% BSA and incubated with 100 μl patient sera diluted 1:300 in 10% normal goat serum (NGS) in phosphate buffered saline (PBS). After washing with PBS 0.05% Tween 20 (PBST), 100 μl goat anti-human IgM-peroxidase conjugate (Cappel, West Chester, Pennsylvania, U.S.A.) diluted 1:4000 in 10% NGS/PBS was added before washing and incubating with 100 μl 0.4 g/l O-phenylenediamine (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) in 0.05 M citrate phosphate buffer, pH 5.0, containing 0.006% hydrogen peroxide. The reaction was stopped at 20 min by the addition of 100 μl of 2.5 M sulfuric acid, and the plate was read with a Multi-skan ELISA reader (Flow Labs, Irvine, Scotland). Samples with an absorbance at 492 nm greater than 0.199 (the mean absorbance plus three standard deviations [S.D.] of 91 healthy Nepali control subjects) were considered positive. Known positive and negative control sera were included on each tray. The variation between assays was less than 10%.
IgG anti-LAM antibodies were measured by ELISA using M. tuberculosis LAM as antigen (kindly supplied by Dr. P. J. Bren-nan) at a concentration of 1 μg/ml and patient sera at 1:1000 dilution (16). Samples with an absorbance at 492 nm greater than 0.419 (mean plus three S.D. of 100 controls) were considered positive.
Antibodies to the M. leprae-specific 35-kDa protein were detected by a monoclonal (ML-04) inhibition ELISA (22,23). Round-bottom microtiter plates (Nunc, Kamstrup, Denmark) were coated with 50 μl of M. leprae sonicate, 10 μg/ml (supplied by IMMLEP), blocked with 3.0% BSA/PBS w/v, and incubated with 25 μ1 serum diluted 1:10, 1:100, 1:1000, 1:10,000 in 1.0% BSA/ PBST for 1 hr. Then 25 μl ML-04-peroxi-dase conjugate (kindly supplied by Dr. J. Ivanyi) diluted 1:400 in 1% BSA/PBST was added for 2 hr. The tray was washed and O-phenylenediamine substrate was added for 30 min. Positive control wells were incubated with conjugate without inhibiting sera. The dilution of the sera causing 50% inhibition of the binding of ML-04 to the M. leprae sonicate (ID50) was calculated and titers greater than 10 were considered positive. All of the 56 control sera tested were negative.
The significance of differences in the se-ropositivity rates between patient groups was tested with the Chi-squared statistic using Yates' correction. The coefficients of correlation among the three assays and between each assay and the BI in individual patients were determined by Pearson's correlation coefficient. The differences in the distribution of absorbances, titers, or BI values between groups were tested by the Mann-Whitney U test; when more than two groups were compared, by the Kruskal-Wallis test.
RESULTS
There were 73 male and 27 female patients with an age distribution from 10 to 73 years. Ten were classified clinically to have BB leprosy, 45 BL, and 45 LL leprosy. For the combined group of MB patients, 89% had IgM anti-PGL-I antibodies, 89% anti-35-kDa antibodies, and 67% IgG anti-LAM antibodies. There were no differences in the seropositivity rates between males and females. The differences in positivity rates and mean antibody levels for the three different classifications of MB disease are illustrated in Table 1. The rises in antibody levels from the BB to LL patient groups were significant for each assay. When the responses to the two M. leprae-specific epitopes were considered together, 90% of BB patients, 89% of BL patients, 100% of LL patients, and 94% of the whole MB group were positive to the PGL-I and/or 35-kDa antigens. Ninety percent were skin-smear positive, and there was a significant rise in the mean BI from the BB to the LL patient groups (Table 1). When the 10 smear-negative patients were analyzed separately, there was no statistically significant difference in the proportion seropositive for any of the three antigens. There was, however, a lower level of IgM anti-PGL-I (p < 0.05) and 35-kDa antibodies (p < 0.05) in the smear-negative MB patients compared to those who were smear positive.
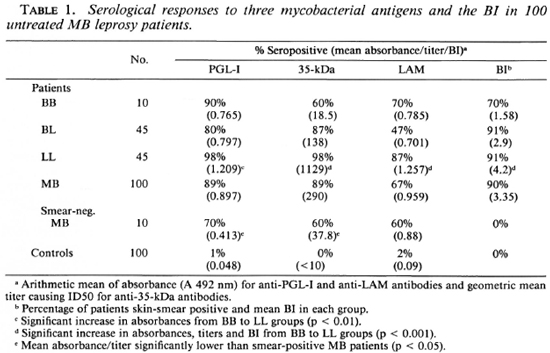
Correlations between antibody assays
MB patients who had detectable anti-35-kDa antibodies were more likely to have IgM anti-PGL-I antibodies (x2 = 19.2, p < 0.001) than to have IgG anti-LAM antibodies (x2 = 3.81, not significant). However, the overlap between the M. leprae-specific antibody responses was not complete. Of the 94 MB patients with antibodies to the 35-kDa antigen and PGL-I, 10 (10.6%) had antibodies to only one of the M. leprae-specific antigens (Fig. 1A). About one third (26 out of 89) of the anti-35-kDa positive patients were IgG anti-LAM negative (Fig. 1B), and a similar proportion (24 out of 89, 26%) of IgM anti-PGL-I positive patients were also anti-LAM negative (Fig. 1C).
Fig. 1. Correlation of antibody levels in individual multibacillary leprosy patients. A = IgM anti-PGL-I (A 492 nm) and anti-35-kDa antibodies (ID50); B = IgG anti-LAM (A 492 nm) and anti-35-kDa antibodies (ID50); c = IgM anti-PGL-I (A 492 nm) and IgG anti-LAM antibodies (A 492 nm).
A comparison of the antibody levels in the three assays showed a moderate correlation between the anti-35-kDa and IgM anti-PGL-I antibodies (Fig. 1A; r = 0.346, p < 0.001) and anti-35-kDa and IgG anti-LAM antibodies (Fig. 1B; r = 0.334, p < 0.001). However, there was no correlation between IgM anti-PGL-I and IgG anti-LAM antibody levels (Fig. 1C; r = 0.052, not significant).
Comparison with clinical parameters
Duration of disease before presentation. Half the patients had disease for 1 year or less before presentation. There were trends for the proportions of patients seropositive to the PGL-I, 35-kDa, and LAM antigens to increase with more longstanding disease and a parallel increase in the mean absorbance or titer (Table 2), but this was statistically significant only for the anti-35-kDa antibodies.
MB patients with established disability at the time of presentation were more likely to be IgM anti-PGL-I positive (95% vs 78%), anti-35-kDa positive (90% vs 86%), and IgG anti-LAM positive (71% vs 59%) than those without disability, but these differences were not significant. There was an increase in the proportion of MB patients seropositive to PGL-I, and a slight increase in the mean IgM anti-PGL-I value with increasing disability. The mean BI did not vary with the period of untreated disease or with the disability index (Table 2).
Clinical extent of disease. When patients without skin infiltration were examined, there was a progressive rise in the proportion of patients seropositive for each assay and in the levels of antibodies as the number of patches increased. However, these changes did not reach statistical significance, except for the IgG anti-LAM antibodies (Table 3). A higher proportion of patients with skin infiltration were positive to any of the three antigens tested compared to patients without infiltration. Mean values for the IgM anti-PGL-I and the anti-35-kDa antibodies and the BI were significantly higher in patients with infiltration.
When the antibody response was compared with nerve involvement (Table 4), there was an increase in seropositivity with increasing numbers of involved nerves, although the changes in antibody levels were variable. The anti-35-kDa and the IgG anti-LAM antibody levels were significantly higher in patients with more nerves clinically involved. There was a rise in the mean BI with an increase in the number of patches and nerves and with the presence of infiltration (Tables 3 and 4).
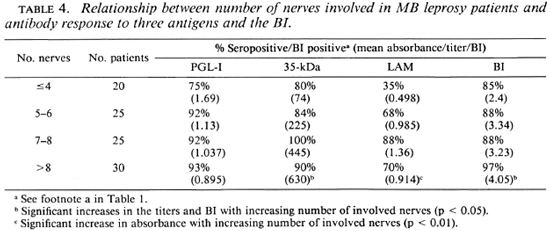
Comparison with BI
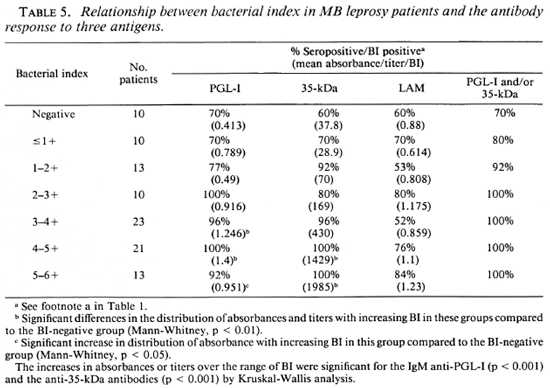
The levels of antibodies to the three antigens were compared with the BI in individual patients (Fig. 2). There was a moderately strong correlation between antibody level and BI for the 35-kDa (r = 0.553, p < 0.001) (Fig. 2A) and the PGL-I antigens (r = 0.34, p < 0.001) (Fig. 2B). The correlation was weaker for the IgG anti-LAM antibodies and the BI (r = 0.199, p < 0.05) (Fig. 2C). When the patients were grouped according to BI levels (Table 5), the progressive and significant rise in the mean level of IgM anti-PGL-I and anti-35-kDa antibodies with increasing BI was apparent. The changes in IgG anti-LAM antibody levels were not significant. All patients with a BI greater than 2.0+ were seropositive to one of the two M. leprae-specific antigens. In all, 8 out of 90 smear-positive MB patients had no detectable IgM anti-PGL-I antibodies, and 7 out of 90 had no anti-35-kDa antibodies. When the two assays were combined, only 3 out of 90 smear-positive MB patients were seronegative. The sensitivity of the IgM anti-PGL-I antibody positivity for detecting smear-positive MB disease was 91%; that of the anti-35-kDa assay, 92% and that of the IgG anti-LAM assay, 68%. The sensitivity of skin smears in detecting MB disease in this group of 100 MB patients was 90%; the sensitivity for each of the two M. leprae-specific antibody assays was 89%.
Fig. 2. Correlation of antibody levels and bacterial index (BI) in individual multibacillary leprosy patients. A = Correlation between anti-35-kDa antibodies (11)50) and Bl (r = 0.553, p < 0.001); B = Correlation between IgM anti-PGL-I (A 492 nm) and 131 (r = 0.34, p < 0.001); C = Correlation between IgG anti-LAM (A 492 nm) and BI (r = 0.199, p < 0.05).
DISCUSSION
The practical value of serological measurements in the control of leprosy has yet to be determined. Initial enthusiasm for the use of M. leprae-specific antibody responses in the serodiagnosis of leprosy has been tempered by negative studies in areas of predominantly paucibacillary leprosy (11) and the recognition that highly specific assays are required to avoid false-positive diagnosis (10). The role of seroepidemiology in those areas in which multibacillary disease predominates or in the contacts of MB patients is the subject of continuing investigation (25). In this study, however, we have addressed the operational value of antibody measurements in patient assessment and, in particular, the contribution of serology compared to the BI. In particular we wanted to examine the relative contributions that skin smears and serological results add to the clinical assessment. Therefore, we have included the 10 smear-negative patients who were considered clinically to be multibacillary. This is important, since the vast majority of field programs have no access to histological diagnosis and if classification is based on a skin smear alone, which is associated with false negatives in the field, the patient may receive inadequate therapy. Interestingly, this small group of smear-negative patients had higher rates of seroposi-tivity and higher levels of antibodies than did patients assigned to the borderline tuberculoid category (21), suggesting that they were not simply paucibacillary patients misclassified.
Both the carbohydrate and peptide M. leprae-specific assays detected 89% of MB patients. Although it has often been implied that all "lepromatous" patients are serologically positive, not all patients are positive to all antigens. In the case of the IgM anti-PGL-I assay, occasional false "negatives" may be due to the presence of anti-BSA antibodies which cause reaction on the control plate. In this study, all negative samples were also negative when retested with a different blocking solution (milk powder) (8). Therefore, a small proportion of MB patients did not respond to the serologically dominant terminal disaccharide present on the ncoconjugate (4,7). In other studies with native PGL-I or various neoconjugates, the proportion of responsive MB patients has been 91% (4), 88%-96% (7,12), and 89% (24).
The response to the M. leprae-specific epitope on the 35-kDa protein showed the greatest gradation in seropositivity and antibody titer from BB to LL patients (Table 1). The 89% positivity rate for this group of MB patients is slightly less than the 98% positivity originally reported by Sinha and coworkers (23). We have previously shown that 33% of Nepali paucibacillary (PB) leprosy patients had anti-35-kDa antibodies, compared to 20% with IgM anti-PGL-I or anti-LAM antibodies (21). The combination of anti-35-kDa and IgM anti-PGL-I assays detected all LL patients and 94% of the MB patient group (Fig. 1), with a moderate correlation between the anti-35-kDa and IgM anti-PGL-I antibody levels in individual patients. A similar correlation had been demonstrated in a group of mainly treated lepromatous patients (19,22).
This concordance between M. leprae-specific assays contrasted with the weaker correlation between seropositivity to LAM and the 35-kDa protein (Fig. 1B) and with the lack of correlation between the anti-PGL-I and anti-LAM antibodies (Fig. 1C). We had included LAM as an antigen because of its strong B-cell immunogenicity (13) and the possibility that it may detect nonresponders to the other antigens. It was, however, associated with the most variable of the responses in untreated MB patients. The LAM antigen employed, although derived from M. tuberculosis, is very similar if not identical to the cell-wall-associated LAM in M. leprae (13). Exposure to environmental mycobacteria and M. tuberculosis in Nepal may have resulted in a higher level of background antibody to LAM in our control subjects and in more variable responses in the leprosy patients. Nevertheless, there was still a significant gradation in mean anti-LAM antibody levels from BB to LL patients (Table 1).
MB leprosy patients have systemic infection involving many sites, not all of which may be clinically apparent. We were interested, however, in determining if the extent of their apparent disease, regardless of the exact classification, correlated with their antibody responses. Patients with more extensive disease, as evidenced by a higher number of skin patches (Table 3), by the presence of skin infiltration or by more involved nerves (Table 4), tended to have a higher proportion of seropositivity and to have higher antibody levels, particularly to the 35-kDa and LAM antigens (Tables 3 and 4). This suggests that increasing antibody responses mirror an increase in the load of disease in the skin and nerves. Using the same clinical groupings, there were parallel increases in the mean BI with increasing skin and nerve involvement (Tables 4-6), confirming the relationship between bacil-lary load and apparent clinical disease. Thus, the increases in antibody levels were an indirect measure of the bacillary load.
When the BI and antibody levels were directly compared in individual patients, the strongest correlation was between the BI and anti-35-kDa antibody (r = 0.553, Fig. 2A) followed by the BI and IgM anti-PGL-I antibodies (r = 0.34, Fig. 2B). When the patients were divided into groups on the basis of their BI, the significant rises in IgM anti-PGL-I levels and in anti-35-kDa titers with increasing BI also were apparent (Table 5). These data are supported by a comparative study between laboratories which showed that the BI and IgM anti-PGL-I antibody levels were significantly related, with correlation coefficients ranging from 0.390 to 0.525 (18). This correlation was less marked if patients with erythema nodosum lepro-sum (ENL) were included since the IgM anti-PGL-I level appeared to fall with acute ENL (17). In a group of mainly treated patients, the anti-35-kDa antibody titer also correlated with the BI (r = 0.458) (22).
The changes in antibody levels with duration of disease, as estimated by the length of untreated disease or the disability index, were less marked. There were also no changes in the mean BI between the categories examined (Table 2). This contrasted with our findings in a series of 154 PB patients (tuberculoid and borderline tuberculoid leprosy) which showed a significant rise in antibody levels to carbohydrate and peptide antigens with increasing disability (21). This may have been due to the observed relationship between antibody levels and the number of nerves involved in tuberculoid disease. Disability was related to the nerves involved and thus to the antibody levels. Therefore, in paucibacillary disease the strongest correlates of antibody response were the number of nerves involved and the subsequent disability. In multibacillary disease, these effects were obscured by the strong response in the majority of patients and the relationship with measurable bacillary load.
The practical application of these studies is whether antibody estimations complement the BI as a routine measure. The poor quality of skin smears in peripheral laboratories (14) and the difficulties in using the BI measurements in control programs (2) have been highlighted recently, and the question raised as to whether M. leprae-specific antibodies offer an alternative (10). Important factors to be considered in this are the sensitivity of the antibody measurements in detecting smear-positive patients, the correlation between the BI on skin-smear and antibody levels in individual patients, and the changes in antibody levels with time. This study does not address the latter issue, which requires longitudinal follow-up studies on cohorts of initially untreated patients to support the findings of cross-sectional studies of patients treated for variable periods of time (1,6,16). In this comparative study, we were able to examine the relative sensitivities of antibody assays and BI in MB patients. The IgM anti-PGL-I assay had a sensitivity of 91% for recognizing smear-positive patients; the sensitivity of the anti-35-kDa assay was 92%. This compared to the sensitivity of anti-LAM assays of 68%. Therefore, the M. leprae-specific assays have similar sensitivities for detecting smear-positive MB patients as well as for detecting all MB patients (89% for each; Table 1). If the sensitivity of the skin smear itself is examined using the clinical diagnosis of BB, BL or LL disease as the benchmark, 10 of 100 patients were negative, giving a sensitivity of 90%. Interestingly, three of these smear-negative MB patients were thought clinically to have infiltrated lepromatous disease, and although the BI was reportedly negative in each patient, the three antibody assays were positive in each patient. This highlights the practical finding that in the field the BI itself is not always a reliable index of apparent lepromatous disease. This discrepancy may be due to an error in the taking or reading of the skin smear, or may be due to misclassification because the patient had BB or BL leprosy rather than LL disease and skin smears had not been taken from correct sites. However, the point is that in an operational sense under field conditions, the BI was negative in 10% of MB patients and had a similar sensitivity for detecting clinical MB disease as did the M. leprae-specific antibody assays. When antibody assays and the smears were used together, the sensitivity for the detection of clinically diagnosed MB disease was 97% for the BI/IgM anti-PGL-I combination and 96% for the BI/35-kDa combination (Table 5).
In conclusion, the levels of M. leprae-spe-cific antibodies and BI parallel the clinical extent of disease in MB patients. There is a moderate correlation between the anti-35-kDa and IgM anti-PGL-I levels and the BI in individual patients, and the combination of antibody levels and the BI increases the sensitivity of detection of MB disease. Our current assessment is that M. leprae-specific antibody levels do not replace but complement the BI in the clinical setting. Whether falls in M. leprae-specific antibodies will parallel the fall in the BI and clinical improvement in a predictable and clinically applicable fashion during multidrug therapy (24) will await the results of further prospective studies. The recently documented fall in the IgM anti-PGL-I antibody levels during clofazimine therapy for dapsone-resistant leprosy suggests that this may be the case (9).
Acknowledgments. The Mycobacterial Research Laboratory is fully supported by The Leprosy Mission (International). We thank Dr. R. J. W. Recs for the provision of M. leprae antigens through the IMMLEP component of the WHO/UNDP/World Bank Tropical Diseases Research Programme; Dr. J. Ivanyi for providing the ML-04 conjugate and Dr. P. J. Brcnnan for providing LAM (prepared under NIH contract Al-52582). This study was dependent on the excellent cooperation of the doctors and the nursing staff at Anandaban Leprosy Hospital, Tansen Hospital, and Green Pastures Hospital. We are grateful to Mrs. Beu-lah Jayakumar for secretarial assistance.
REFERENCES
1. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-l and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. Becx-Bleumink, M. Operational aspects of multidrug therapy. Int. J. Lepr. 57(1989)540-551.
3. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from M. leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
4. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugates containing the M. leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-83.
5. Britton, W. J., Garsia, R. J. and Basten, A. Serological response to phenolic glycolipid of Mycobacterium leprae in Australian and Nepali leprosy patients. Aust. N.Z. J. Med. 17(1987)568-573.
6. Britton, W. J., Hellqvist, L., Basten, A. and Raison, R. L. Mycobacterium leprae antigens involved in human immune responses. 1. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
7. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
8. Douglas, J. T., Hirsch, D. S., Fajardo, T. T., Cellona, R. V., Abalos, R. M., dela Cruz, E. C, Madarang, M. G., de Wit, M. Y. L. and Klatser, P. R. Evaluation of Mycobacterium leprae antigens in the serological monitoring of a clo-fazimine-based chemothcrapeutic study of dap-sone resistant lepromatous leprosy patients in Cebu, Philippines. Lepr. Rev. 60(1989)8-19.
9. Douglas, J. T., Wu, Q.-X., Agustin, G. P. and Madarang, M. G. Evaluation of inexpensive blocking agents for ELISA in the detection of antibody in leprosy. Lepr. Rev. 59(1988)37-44.
10. Fine, P. E. M. Immunological tools for leprosy control. Int. J. Lepr. 57(1989)671-686.
11. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. C. Seroepide-miological studies of leprosy in northern Malawi based on enzyme linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
12. Fujiwara, T., Hunter, S. W., Cho, S.-N., Aspi-nall, G. O. and Brennan, P. J. Chemical synthesis and serology of disaccharides and trisac-charides of phenolic glycolipid antigens from the leprosy bacillus preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
13. Gaylord, H. and Brennan, P. J. Leprosy and the leprosy bacillus; present developments in characterization of antigens and immunology of the disease. Ann. Rev. Microbiol. 41(1987)645-675.
14. Georgiev, G. D. and McDougall, A. C. Skin smears and the bacterial index (BI) in multiple drug therapy leprosy control programs: an unsatisfactory and potentially hazardous state of affairs. (Letter) Int. j. Lepr. 56(1988)101-103.
15. Klatser, P. R., de Wit, M. Y. L. and Kolk, A. H. J. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 58(1985)468-473.
16. Levis, W. R., Meeker, H. C, Schuller-Levis, G. B., Sersen, E., Brennan, P. J. and Fried, P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J. Infect. Dis. 56(1987)763-769.
17. Lyons, N. F., Shannon, E. J., Ellis, B. P. B. and Naafs, B. Association of IgG and IgM antibodies to phenolic glycolipid antigen of M. leprae with disease parameters in multibacillary leprosy patients. Lepr. Rev. 59(1988)45-52.
18. Meeker, H. C, Levis, W. R., Sersen, E., Schuller-Levis, G., Brennan, P. J. and Buchanan, T. M. ELISA detection of IgM antibodies against phenolic glycolipid-I in the management of leprosy: a comparison between laboratories. Int. J. Lepr. 54(1986)530-539.
19. Mwatha, J., Moreno, C, Sengupta, U., Sinha, S. and Ivanyi, J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr. Rev. 59(1988)195-199.
20. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
21. Roche, P. W., Britton, W. J., Failbus, S. S., Ludwig, H., Theuvenet, W. J. and Adiga, R. B. Heterogeneity of serological responses in pauci-bacillary leprosy-differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58(1990)319-327.
22. Sinha, S., McEntegart, A., Girdhar, B. K., Bhatia, A. S. and Sengupta, U. Appraisal of two Mycobacterium /e/)rae-spccific serological assays for monitoring chemotherapy in lepromatous (LL/ BL) leprosy patients. Int. J. Lepr. 57(1989)24-32.
23. Sinha, S., Sengupta, U., Ramu, G. and Ivanyi, J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int. J. Lepr. 53(1985)33-38.
24. WHO Study Group. Chemotherapy of leprosy for control purposes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
25. Workshop on Immunology of leprosy, XIII International Leprosy Congress. Int. J. Lepr. 57Suppl.(1989)275-277.
26. Young, D. B.,and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. B.App.Sc; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
2. Ph.D., F.R.A.C.P., F.R.C.P.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
3. M.Sc; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
4. M.D.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
5. M.D.; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
6. M.D., M.P.R.C.H., Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
Present address for Dr. Britton: Clinical Immunology Research Centre, University of Sydney, Sydney 2006, Australia.
Present address for Dr. Williams: Tan-sen Hospital, Palpa, Nepal.
Present address for Dr. Pradhan: Leprosy Control Section, Public Health Division, Ministry of Health, Teku, Kathmandu, Nepal.
Reprint requests to Paul W. Roche.
Received for publication on 11 October 1989.
Accepted for publication in revised form on 26 March 1990.