- Volume 58 , Number 3
- Page: 491–502
Quantitation of IgM antibodies to the M. leprae synthetic dissacharide can predict early bacterial multiplication in leprosy
ABSTRACT
Quantitative enzyme-linked immunosorbent assays detecting IgM to the soluble Mycobacterium leprae crude sonicate (CD75) and the synthetic disaccharide antigen coupled to bovine serum albumin (ND-BSA) were assessed for their ability to determine early infection in families/household contacts of leprosy patients and employees of a leprosy center working in close contact with leprosy patients. Although IgM to both antigens (CD75 and ND-BSA) correlated with the bacterial index (BI) assessed histologically on skin-biopsy samples, the level of IgM antibodies to ND-BSA was a much more sensitive indicator of low bacterial loads. A 4.4-fold difference in antibody levels was observed between the mean group levels of endemic controls (N = 116) and tuberculoid leprosy patients with a BI of 0 (N = 88), increasing to sevenfold in tuberculoid leprosy patients with a BI of 1 (N = 20). Using a statistical cut off with endemic controls (mean + 2 S.D.), household/family contacts showed 30% seropositivity (N = 180) as compared to staff contacts who showed 17% seropositivity (N = 55). Percent seropositivity in family contacts was not related to the type of leprosy of the index case (lepromatous vs. tuberculoid) or the duration of treatment of the index case. Age of the individual in the family contact group had a significant influence on seropositivity. These results support the hypothesis that, in this community, factors other than the viable bacterial load of the index case, such as genetic susceptibility, may be influencing the high rate of seropositivity in family contacts. IgM ND-BSA antibodies seem to provide a good indicator of low antigenic loads and could prove to be useful in detecting subclinical infection before the onset of dis-ease. Follow-up studies of these seropositive individuals are in progress to understand the relationship between seropositivity and the progress of clinical disease.RÉSUMÉ
Des titrages enzymatiques quantitatifs (ELISA) pour la détection d'IgM vis-à-vis du sonicat brut soluble (GD75) de Mycobaclerium leprae et l'antigène disac-charide synthétique couplé à l'albumine sérique bovine (ND-BSDA) furent évalués quant à leur capacité de révéler précocement l'infection parmi des contacts familiaux/domiciliaires de patients lépreux et des employés d'un centre de lèpre travaillant en contact étroit avec des patients. Bien que les IgM vis-à-vis des deux antigènes (CD75 et ND-BSA) étaient en relation avec l'indice bactériologique (IB) évalué histologiquement sur des échantillons de biopsies cutanées, le niveau des anticorps IgM vis-à-vis de ND-BSA était un indicateur beaucoup plus sensible des faibles charges bactériennes. Une différence d'un facteur de 4,4 fut observée entre les taux moyens d'anticorps du groupe témoin de milieux endémiques (N = 116) et les lépreux tub-erculoïdes ayant un IB égal à 0 (N = 88); cette différence augmente à sept fois pour les lépreux tuberculoïdes ayant un IB de 1 (N = 20). Utilisant un seuil statistique par rapport aux témoins des milieux endémiques (moyenne + 2 S.D.), les contacts familiaux/domiciliaires ont montré 30% de séropositivité (N = 180), par rapport au personnel en contact avec les patients, qui ont montré une séropositivité de 17% (N = 55). Le pourcentage de positivité parmi les contacts familiaux ne dépendait pas du type de lèpre du cas index (lépromateux vs tuberculoïde) ni de la durée du traitement du cas index. L'âge des individus dans le groupe des contacts familiaux avait une influence significative sur la séropositivité. Ces résultats supportent l'hypothèse que, dans cette communauté, des facteurs autres que la charge bactérienne viable du cas index, tels que la susceptibilité génétique, peuvent influencer le taux élevé de séropositivité parmi les contacts familiaux. Les anticorps IgM vis-à-vis de ND-BSA semblent fournir un bon indicateur de charges antigéniques faibles, et pourraient se révéler utiles dans le détection de l'infection subclinique avant l'éclosion de la maladie. Des études de suivi de ces individus séropositifs sont en cours, dans le but de comprendre la relation entre la séropositivité et le développement de la maladie clinique.RESUMEN
Se usaron ensayos inmunoenzimáticos para detectar la infección leprosa temprana en base a la presencia de IgM contra un sonicado crudo (CD75) de Mycobacterium leprae y contra el disacárido sintético acoplado a albúmina sérica bovina (ND-BSA) en el suero de convivientes y familiares en contacto con pacientes con lepra y en el suero de empleados de un centro lepro-lógico en estrecho contacto con los pacientes. Aunque los anticuerpos IgM contra ambos antigenos (CD75 y ND-BSA) correlacionaron bien con el índice bacteriano (BI) establecido por el estudio histológico de las biopsias de piel, el nivel de anticuerpos contra el ND-BSA fue el indicador más sensible cuando las cargas bacterianas fueron bajas. Se observó una diferencia de 4.4 veces en los niveles de anticuerpos entre los controles endémicos (N = 116) y los pacientes con lepra tuberculoide con un BI de 0 (N = 88), y de 7 veces en los pacientes tubereuloides con un BI de 1 (N = 20). Haciendo las correcciones estadísticas en relación a los controles endémicos (media + 2 D.E.), los convivientes y familiares mostraron un 30% de seropositividad (N = 180) en comparación con los contactos del centro lcprológico quienes mostraron una seropositividad del 17% (N = 55). El porcentaje de seropositividad en los contactos familiares no estuvo relacionado con el tipo de lepra del caso índice (lepromatoso vs tuberculoide) ni con la duración de su tratamiento. La edad de los individuos en el grupo de contactos familiares tuvo una influencia importante en la seropositividad. Estos resultados apoyan la hipótesis de que, en esta comunidad, otros factores diferentes a la carga bacteriana viable, tales como la susceptibilidad genética, pueden influir en la alta frecuencia de seropositividad en los contactos familiares. Los anticuerpos IgM anti-ND-BSA, parceen scr un buen indicador de bajas cargas antigénicas y pueden resultar utiles en la detección de la infección subclínica antes de que la enfermedad se haga aparente. Los estúdios de seguimiento de estos indivíduos seropositivos para establecer la relación entre seropositividad y progreso de la enfermedad, se encuentran actualmente en desarrollo.Early diagnosis and chemotherapeutic intervention is the most essential prerequisite for decreasing deformities associated with leprosy. Early chemotherapeutic intervention should also lead to interruption of disease transmission. The etiological agent, Mycobacterium leprae, is a slow-growing bacterium (10-12 days doubling time), and therefore requires a long incubation time before it attains the diagnostically detectable load in the skin (104 per gram of tissue) (6). Initiation of antibody responses generally requires a much lower antigenic load and, therefore, should precede the detection of bacteria in the skin. Thus antibodies, particularly of the IgM isotype, should aid in the early diagnosis of infection in leprosy. However, leprosy antibody detection using a crude M. leprae sonicate has never attained the desired specificity (1,14). This problem was not resolved with the availability of sensitive radioimmunoassays and enzyme-linked antibody assays (15,21)
The M. leprae-specific antigen era started with the purification of a novel phenolic glycolipid (PGL-I) from cell walls (3,12) and recognition of the terminal sugars of PGL-I as the immunodominant species-specific determinant (10). The sugars were synthesized and attached to bovine serum albumin to render them immunologically reactive in antibody assays. Both PGL-I and the synthetic sugars were shown to react with IgM in a highly specific manner in patients with leprosy (4,5,8,25). This was very encouraging since IgM antibodies have been a very useful indicator of early or acute infection in several infectious diseases.
Three recent studies have looked at the serological usefulness of these highly purified and synthetic antigens in detection of early infection in different leprosy-endemic regions (7,9,16) by assessing IgM antibodies in the group most at risk of exposure and infection -household contacts of leprosy patients. The method of choice for detecting antigen-specific antibodies has been the enzyme-linked immunosorbent assay (ELISA) (22) because of its sensitivity as well as its capability of handling large numbers of sera simultaneously. In Malawi (9), seropositiv-ity was established on the basis of group means of nonendemic control sera. A cut-off value of 0.16 optical density for a dilution of 1 /250 resulted in 98% specificity with multibacillary patients. In the Ethiopian population (16) a standard dilution of 1/100 was used, but instead of using a population-based cut-off value, the activity of test sera was expressed relative to a lepromatous serum pool. An activity of less than 6% was considered negative. Both of these studies conducted in populations with wide variations in leprosy prevalence were unable to show differences in seropositivity among the endemic population and household contacts.
In French Polynesia (7) a total of 850 household contacts were tested with the three available synthetic sugars. Again, a single serum dilution of 1/250 was used. Using a much higher optical density (0.500) cut off, based on an endemic population mean (mean + 2 S.D.), a seropositivity of 19.5% in household contacts was reported. This was an extremely stringent cut off since only 21% of the tuberculoid patients were positive by this criteria. Upon follow up, three seropositive individuals developed clinical leprosy within 2 years. Although the numbers arc small, this study does show that early infection was being detected serologically, which may eventually lead to clinical disease if additional cellular protective mechanisms arc not activated.
In the above studies, it is impossible to compare the results among populations or investigators since the results are expressed as optical density readings and seropositivity is based on widely differing control populations. Attempts to standardize PGL-I ELISA using the synthetic dissacharide attached to bovine scrum albumin (D-BSA) (20) is certainly a step in the right direction. With the availability of reference sera from the World Health Organization (WHO) for these antigens, comparison among populations would become a reality.
Some of the above limitations can be overcome by expressing the activity of an individual serum relative to a reference serum with assigned units of activity (11). This allows for greater standardization and quantitation in addition to having the distinct advantage of being able to relate values obtained in different assays, as well as by different investigators and laboratories. We have now further evaluated this quantitative IgM D-BSA ELISA for its ability to assess the antigenic load in clinical leprosy and predict early bacillary multiplication in household contacts of leprosy patients residing in Karachi, a city of over 8 million in Pakistan, having the highest proportion of leprosy patients in the country. Pakistan has about 30,000 registered cases of leprosy. Of these, 61% reside in Karachi which has a prevalence of 3‰ (17).
MATERIALS AND METHODS
Patient material. All of the clinical material was obtained from the Marie Adelaide Leprosy Centre in Karachi, Pakistan, the main referral center with a 100-bed inpatient facility which registers 500-600 new patients annually. In addition to the main referral center, there arc nine subcenters affiliated with the main center in Karachi.
The study groups consisted of: a) One-hundred-eighty-six (186) untreated leprosy patients, diagnosed clinically and histologically confirmed by one of us (SL). A punch skin biopsy (4-mm) was taken for determination of the type of leprosy and the bacterial index (BI). The BI was assessed histologically using Ridley's scale (19). Two-to-five Wade-Fite-stained sections were examined for acid-fast bacilli. The patients were classified into the following groups based on histology: Lepromatous (polar and subpolar) (LL), N = 29; borderline lepromatous (BL), N = 31; borderline (BB), N = 6; borderline tuberculoid (BT), N = 84; tuberculoid (primary and secondary) (TT), N = 16, and indeterminate (I), N = 20.
b) One-hundred-eighty (180) family/ household contacts of leprosy patients with no evidence of clinical leprosy. These families came from four different areas within 5-10 kilometers of the main referral center and The Aga Khan University. The index case had been diagnosed clinically, and the BI was established by slit-skin smears. Diagnosis of all index cases with under 1 year of treatment was also confirmed histologically.
c) Fifty-five (55) healthy staff members working at the Marie Adelaide Leprosy Centre (clinicians and leprosy technicians) for 2-25 years with no clinical signs of leprosy.
d) One-hundred-sixteen (116) endemic controls consisting of random blood donors presenting at the hospital, employees, and students at The Aga Khan University/Hospital. The mean age of this group was not statistically different from that of the contacts. For obvious reasons, we could not request a family history of leprosy from these donors. However, this group represented all areas of Karachi. In addition, 47 patients presenting to a community clinic for reasons other than leprosy and more closely representing the socioeconomic level of the study group were also included as a second control group.
Five-to-ten ml of blood was collected in venoject tubes. The samples were kept at room temperature for 1-2 hr and then at 4ºC overnight before centrifugation in order to separate the serum. The sera were stored in small aliquots (0.1 ml) at - 70ºC until used.
Antigens. Both armadillo-derived M. leprae, soluble sonicate antigen (batch CD75) and the synthetic disaccharide co-valently coupled to bovine serum albumin (D-BSA) were kindly provided by Dr. R. J. W. Rees, National Institute for Medical Research, London. The antigen stocks were stored at - 70ºC as small aliquots until used.
Reagents. Alkaline phosphatase conjugates of anti-human IgM and IgG minus horse and bovine reactivity (Jackson Laboratories, Avondale, Pennsylvania, U.S.A.) were used throughout the study. Bovine scrum albumin (BSA), Tween 20, and the substrate for alkaline phosphatase were purchased from Sigma Chemical Co., Poole, U.K.
ELISA. The optimization of a quantitative ELISA for both the crude sonicate antigen of M. leprae and the synthetic disaccharide has been described in detail previously (11). The only modification was the use of Immulon 2 microtitcr plates (Dy-natech, Alexandria, Virginia, U.S.A.). Briefly, plates were coated with 1 μg/ml of D-BSA or 4 μg/ml of CD75 in 0.1 ml of carbonate buffer (0.05 M) at pH 9.6. The plates were washed and blocked with 5% BSA as described previously. Test sera were incubated at four serial fourfold dilutions ranging from 20-1280 for tuberculoid leprosy patients, contacts, and endemic controls, and 80-5120 for lepromatous leprosy patients. Antibody conjugates and substrate were used at the manufacturer's recommended concentrations.
Preparation of a reference serum pool and assignment of units of activity has also been described in detail elsewhere (11). This pool was derived from high-titered sera of untreated lepromatous leprosy patients. The reference serum pool was assigned 6,400 units for IgM D-BSA and 51,200 units for IgM CD75 after establishing full titration curves against the relevant antigen. A nine-point calibration curve in duplicate was generated for the reference pool using twofold serum dilution in each assay. In addition, two internal standards (corresponding to midpoint and endpoint of the calibration curve) were also run in each assay to determine the interassay coefficient of variation. The test sera were assigned units of activity using this standard calibration curve (Fig. 1).
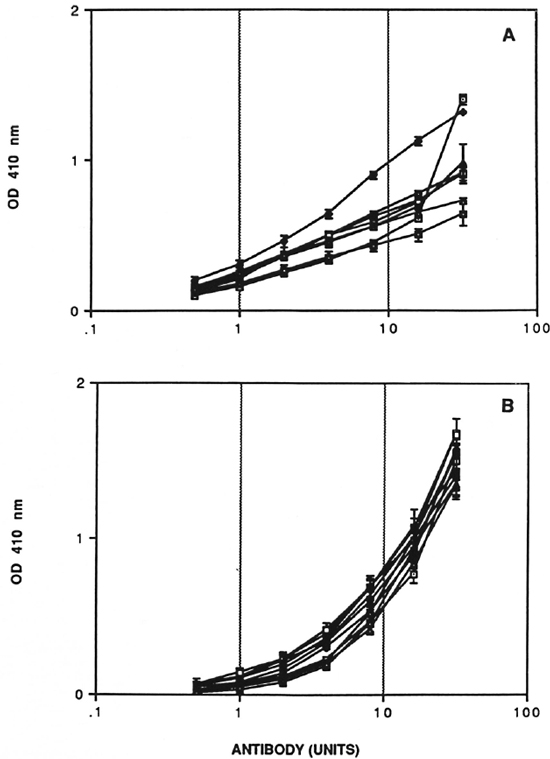
Fig. 1. Calibration curve with reference scrum pool. Dose response curves for IgM CD75 (A) and IgM D-BSA (B) units of antibodies. Standard errors for plate-to-plate variation (N = 3) at each point are indicated.
Statistics. Descriptive, linear regression (r) and multiple regression (r2) analyses were carried out using the Statworks® program on an Apple Macintosh Plus microcomputer. Antibody levels in different groups were compared by unpaired t tests.
RESULTS
Quality control studies. The quantitative ELISA for IgM D-BSA and IgM CD75 was rigorously characterized in terms of performance characteristics. Experiments were performed to evaluate the reproducibility and precision of calibration curves. A minimum of three to four plates with standard calibration curves were run in each assay. The standard errors at each individual concentration for calibration curves run simultaneously are indicated by horizontal error bars, and the results for eight IgM anti-CD75 and ten IgM anti-D-BSA assays are shown in Figure 1. There was very little variation from plate to plate within the same assay for both systems. The most important feature of the IgM D-BSA calibration curve was the low noise or background level in the lower range of the curve, allowing accurate assessment of low antibody activity; an important prerequisite for analysis of paucibacillary leprosy patients and household contacts of leprosy patients. To analyze the intra- and interassay coefficient of variation of the dose response curve, both linear (r) and multiple regression (r2) analysis was carried out as shown in Table 1. The interassay coefficient of variation (CV) for IgM D-BSA was 2.5% (r2 = 0.97) as compared to 9.2% (r2 = 0.848) for IgM CD75, indicating that the D-BSA values were much more comparable between assays.
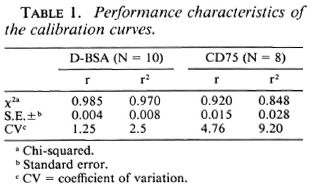
Correlation of IgM antibodies with BI. Patients in the entire clinical spectrum of leprosy (186) were divided on the basis of their Bis as assessed histologically on 4-mm punch biopsies of skin. Figure 2 shows the mean group levels of IgM D-BSA and IgM CD75 with increasing BIs. A monotonic linear relationship was obtained with both antigens. Both IgM CD75 and IgM D-BSA showed significant differences between the endemic controls and clinically and histologically diagnosed tuberculoid leprosy patients with a BI ofO. IgM D-BSA showed a fourfold difference between these groups, while IgM CD75 showed a rise of only 1.6-fold. These results indicate that low levels of bacteria, not detected histologically by examining 2-5 sections per biopsy, are evoking significant IgM D-BSA responses. This could be used in the diagnosis of early bacterial multiplication. Individuals with a BI of 1 showed a sevenfold higher level of IgM D-BSA than the endemic controls. It was interesting to note that the IgM D-BSA antibodies tend to plateau above a BI value of 3 while antibodies to the crude antigens arc still rising which may indicate that with an increase in the bacterial load additional antigens represented in the crude sonicate may also be evoking IgM responses.

Fig. 2. Relationship between bacterial index determined histologically in leprosy patients and units of IgM antibodies to D-13SA (A) and CD75 (B). Antibody levels in healthy endemic controls (EC) are also indicated for comparison. The mean of each group is given and the vertical bars indicate the standard error around the group mean; figures in parentheses arc number in each group.
Distribution of IgM D-BSA in contacts of leprosy patients. Since IgM D-BSA antibody levels showed a much greater degree of sensitivity with a low bacillary load, we have only reported the levels of IgM D-BSA antibodies in leprosy patients and contacts of leprosy patients.
The distribution of IgM D-BSA in the household contacts of lepromatous (N = 70) and tuberculoid (N = 110) leprosy patients and the staff/employees (N = 55) of a leprosy center in close contact with leprosy patients is shown in Figure 3. For comparison, the levels in an age-matched endemic population (N = 116) are also indicated. A high proportion of both household and staff contacts showed significantly higher levels of antibodies than did the endemic individuals (mean + 2 S.D.), which was subsequently used as a cut-off point for expressing sero-positivity.
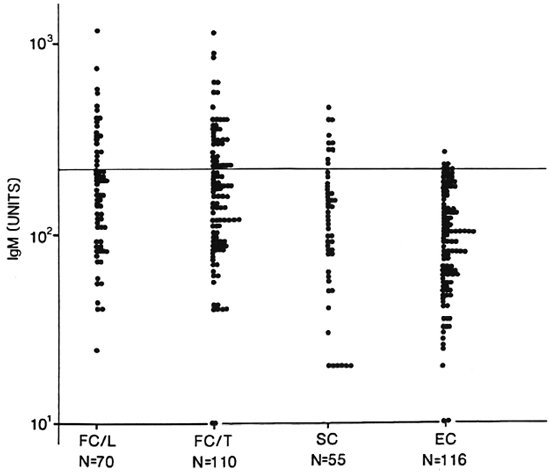
Fig. 3. Distribution of IgM antibodies in healthy family contacts (FC) and staff contacts (SC) of leprosy patients and endemic controls (EC). Each point represents an individual. Groups were divided according to the index case [lepromatous (L) or tuberculoid (T)]. Classification was done clinically and, in some cases, histologically. N = number tested in each group; horizontal line = statistical cut off for seropositivity.
Table 2 shows the percent seropositivity in both leprosy patients (N = 160) and contacts of leprosy patients. (Patients in the indeterminate and borderline classification groups are not included in this table.) Patients with lepromatous disease showed a 100% seropositivity as expected due to their high bacterial load. What is surprising is that even with the stringent cut-off using endemic controls group values, the rate of seropositivity in both the BT (57%) and TT (40%) group is significantly higher than shown in earlier studies using ELISA methodology (7,9,16). When contacts of leprosy patients were similarly analyzed, the seropositivity (30%-35%) was slightly lower than the tuberculoid leprosy patient group. The staff contact group showed a seropositivity of 17%, twofold lower than the family contacts.
Effect of duration of treatment of index case. Since disease transmission is only possible with patients containing viable bacteria (untreated and short-term treated), we also looked at seropositivity in family contacts in relation to the length of time the index case had been on chemotherapy.
Figure 4A shows the distribution of IgM D-BSA in relation to the duration of treatment of the index case at the time the family contacts were tested. No significant differences were seen in the rate of seropositivity in contacts of leprosy patients with duration of chemotherapy of the index case. The rate of seropositivity in household contacts where the index case had been treated for under 90 days and would, therefore, have more viable bacteria, was the same as for those treated for longer periods. The same was true of both the lepromatous and the tuberculoid leprosy patients, again indicating that seropositivity in family contacts in this community was related to factors other than just the bacterial load of the index case.
There was a trend with age of seropositivity to D-BSA (Fig. 4B) as reported by other investigators (9). The greatest seropositivity was seen with individuals under 30 years of age. The higher age group (> 40) needs to be expanded further to perform meaningful statistical analysis.
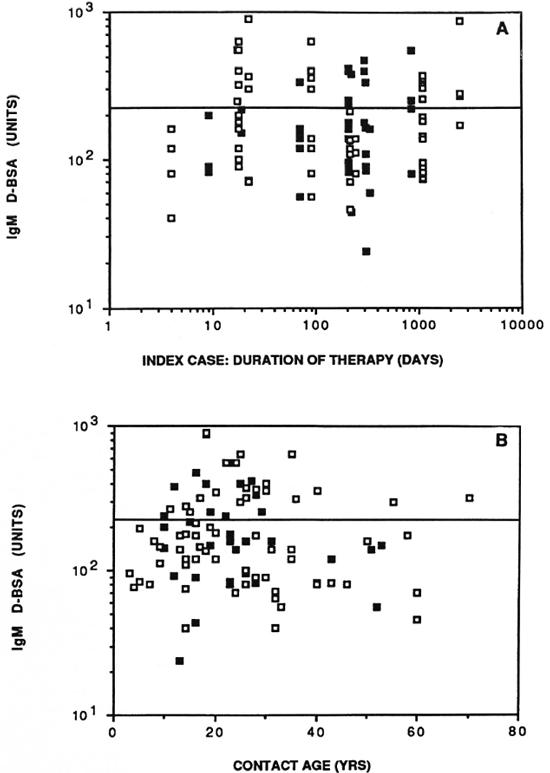
Fig. 4. Antibody activity in household contacts in relation to duration of chemotherapy of the index case in days (A) and age of the contact in years (B).  = contacts of lepromatous leprosy patients;
= contacts of lepromatous leprosy patients;  = contacts of tuberculoid leprosy patients; horizontal line = statistical cut off for seropositivity.
= contacts of tuberculoid leprosy patients; horizontal line = statistical cut off for seropositivity.
DISCUSSION
The antibody levels which indicate subclinical infection using either crude or purified antigens based on ELISA methodology have not been thoroughly established. Most of the earlier studies have utilized a semiquantitative ELISA using optical density readings with single point readings. We have further refined the methodology by providing a more quantitative determination of the antibody levels to both the crude sonicate antigen as well as the synthetic disac-charide (D-BSA).
To evaluate if IgM antibodies correlate with the bacterial load, untreated leprosy patients were divided into groups on the basis of their Bis. A detailed analysis of the distribution of the IgM antibodies showed a 4.4-fold difference in mean IgM D-BSA levels of histologically confirmed tuberculoid leprosy patients with a BI of 0 as compared to the endemic controls (p < 0.001). This difference increased to sevenfold in patients with a BI of 1. Differences in IgM antibodies to the crude sonicate were considerably less in both of these groups (BI = 0 and 1) compared to endemic controls (Fig. 2). The low level of IgM antibody binding with the crude sonicate may be due to the fact that PGL-I evokes the predominant IgM response in individuals with low bacterial loads but is largely removed during the processing of M. leprae before sonication (21). At higher bacterial loads, the antigens in the crude sonicate arc inducing considerable IgM antibodies while the PGL-I response is beginning to plateau. Thus, IgM responses to PGL-I could provide a more sensitive indicator of early bacterial multiplication. The obvious advantage of using D-BSA rather than PGL-I is its high solubility in aqueous solutions which results in better reproducibility and linearity of the antibody binding. Since a high degree of correlation exists between the binding of IgM to PGL-I and D-BSA (24), we have evaluated only IgM D-BSA responses in household and staff contacts.
To determine the rate of seropositivity, a statistical cut off (mean + 2 S.D.) was established with an endemic control group selected from volunteers from among the students and employees/staffof The Aga Khan University, as well as random blood donors at the University hospital which is situated 5 kilometers from the main referral center. Karachi has a high rate of prevalence, and most of the areas within 10 kilometers of the institution (which is where the majority of the volunteers reside) arc highly endemic for leprosy. However, this group may represent a slightly higher socioeconomic level. Therefore, a second control group was selected from patients presenting at a community clinic at The Aga Khan Hospital for reasons other than leprosy. This clinic is specially subsidized for low income groups and was included to control for the socioeconomic level. No difference in seropositivity was observed between the two control groups.
Among the leprosy contact group, 32% seropositivity was observed within the household contact group and 17% within the staff contacts. The seropositivity rates observed in most contact populations arc much higher (7,16,22) than the prevalence of clinical leprosy reported in the contact population (13,18). This is not surprising, since all individuals who are seropositive will not go on to develop clinical leprosy. The genetic susceptibility of an individual in terms of developing protective cellular immune mechanisms will eventually determine self-healing or progression to clinical disease. However, in our study, greater than 500 units of antibody to D-BSA in an individual indicates considerable bacterial multiplication and bacterial load, as shown in Figure 2. There were 12 such individuals in the household contacts where the IgM antibodies were 500 units or greater. Fifty percent of these did not show any significant levels of IgG antibodies (data not shown) which is considered to be an indicator of T-cell activation. Individuals with a tendency toward developing lepromatous disease would be most likely to show such early high levels of IgM antibodies, and they should be considered for chemotherapy. Unless early detection and effective chemotherapy is provided at this level, it would be difficult to interrupt transmission in highly endemic areas despite effective drugs for treatment.
The type of leprosy of the index case (lepromatous vs. tuberculoid) in the family did not have an influence on the rate of seropositivity to D-BSA in the family contacts. This was surprising, since earlier studies report both a higher incidence of clinical disease (18) and a higher rate of seropositivity to D-BSA in contacts of lepromatous leprosy patients (7). This may reflect that additional factors such as population density and prevalence of disease within the community may be diluting out differences due to the bacterial load of the index case. In such communities, genetic susceptibility may become an overriding determinant of clinical disease. This is supported to some extent by the analysis of seropositivity in contacts of leprosy patients in relation to the duration of treatment. If transmission was related to the presence of viable bacterial load in the index case, a higher incidence of seropositivity should have been observed in contacts of recently treated patients than that in those who have been on long-term chemotherapy.
It was interesting to note that family/ household contacts showed a twofold higher rate of seropositivity than the staff contacts. This could not be attributed to differences in age distribution in the two groups since most of the staff workers were between the ages of 20-40 where the highest rate of seropositivity occurs (Fig. 4B). This twofold difference may again reflect an influence of genetic factors associated with increased susceptibility in family/household contacts as compared to staff contacts. Annual serological and clinical followup is in progress in this group of family/household contacts to determine the relationship between seropositivity and the development of overt leprosy disease.
The strongest evidence available for genetic factors in determining host resistance to mycobacterial infections has been provided in studies of the mouse model (reviewed in 2). Collaborative studies for probing at the gene level for mycobacterial susceptibility genes in families with multiple cases of leprosy patients have already been initiated.
Acknowledgments. This investigation received financial support from the UNDP/World Bank/WHO Spécial Programme for Research and Training in Tropical Discascs and a grant from The Rockefeller Foundation.
Sincere thanks arc expressed to Dr. Pfau, Dr. Thomas Chiang, and to the Marie Adelaide staff for their continuing support. Special thanks arc due to Mr. Mustapha Ali Khan for his help in the field work. We would also like to thank Dr. Ulmer, Dean, The Aga Khan University, for his encouragement and support to our program. Continued support by Professor Keith McAdam, Department of Clinical Sciences, London School of Tropical Hygiene and Medicine, is sincerely acknowledged.
The excellent technical assistance by Ms. Maqboola Dhojki, Fareeza Samad, and Mr. Neville Lyall in processing and cataloging of clinical material is gratefully acknowledged.
Finally, we would like to thank Dr. Rees, National Institute for Medical Research, London, for the supply of M. leprae antigens.
REFERENCES
1. ABE, M., Minaoawa, F., Yoshino, Y., Ozawa, T., Saikawa, K. and Saito, T. Fluorescent leprosy antibody absorbtion (FLA-ABS) test for detecting sublinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
2. Blackwell, J. M. Bacterial infections. In; Genetics of Resistance to Bacterial and Parasitic Infections. Wakelin, D. and Blackwell, J. M., eds. London: Taylor and Francis, Ltd., 1988.
3. Brennan, P. J. and Barrow, W. W. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int. J. Lepr. 48(1980)382-387.
4. Brett, S. J., Draper, P., Payne, S. N. and Rees, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immun. 52(1983)271-279.
5. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugatcs containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid 1 in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
6. Bryceson, A. and Pfaltzgraff, R. E. Leprosy. 2nd ed. London: Churchill Livingstone, 1979, p. 34.
7. Chanteau, S., Cartel, J.-L., Roux, J., Pliciiart, R. and Bach, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid I in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-775.
8. Cho, S.-N., Fujiwara, T., Hunter, S. W., Rea, T. H., Gelber, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3, 6-di-O-methyl-β-d-glucopyranosyl epitope for the sero-diagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
9. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. C. Serocpide-miological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
10. Fujiwara, T., Hunter, S. W., Cho, S.-N., Aspi-nall, G. O. and Brennan, P. J. Chemical synthesis and serology of disaccharides and trisac-charides of the phenolic glycolipid antigens from the leprosy bacillus and preparation of a disac-charide protein conjugate for serodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
11. Hasan, R., Dockrell, H. M., Chiang, T. and Hussain, R. Quantitative antibody ELISA for leprosy. Int. J. Lepr. 57(1989)766-776.
12. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
13. Jesudasan, K., Bradley, D., Smith, P. G. and Christian, M. Incidence rates of leprosy among household contacts of primary cases. Indian J. Lepr. 56(1984)600-614.
14. Ji, B.-H., Tang, Q.-K., Li, Y.-L., Chen, J.-K., Zhang, J.-L., Dong, L.-W., Wang, C.-M., Ma, J.-J. and Ye, D.-L. The sensitivity and specificity of fluorescent leprosy antibody absorbtion (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Lepr. Rev. 55(1984)327-335.
15. Melsom, R., Harboe, M., Myrvang, B., Godal, T. and Belehu, A. Immunoglobulin class specific antibodies to M. leprae in leprosy patients, including the indeterminate group and healthy contacts, as a step in the development of methods for serodiagnosis of leprosy. Clin. Exp. Immunol. 47(1982)225-233.
16. Menzel, S., Harboe, M., Bergsvik, H. and Brennan, P. J. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
17. Pfau, R. and Haq, G. Leprosy in Pakistan. Lepr. Rev. 57(1986)355-359.
18. Rao, P. S. S., Karat, A. B. A., Kaliaperumal, V. G. and Karat, S. Transmission of leprosy within households. Int. J. Lepr. 43(1975)45-49.
19. Ridley, D. S. Skin Biopsy in Leprosy. 2nd ed. Basle: CIBA-GEIGY Ltd., 1985.
20. Sanchez, G. A., Malik, A., Tougne, C, Lambert, P. H. and Engers, H. D. Simplification and standardization of sérodiagnostic tests for leprosy based on phenolic glycolipid-1 (PGL-1) antigen. Lepr. Rev. 57(1986)83-93.
21. Touw, J., Langendijk, E. M. J., Stoner, G. L. and Belehu, A. Humoral immunity in leprosy: immunoglobulin G and M antibody responses to Mycobacterium leprae in relation to various disease patterns. Infect. Immun. 36(1982)885-892.
22. Voller, A. and de Savigny, R. Diagnostic serology of tropical parasitic diseases. J. Immunol. Methods 46(1981)1-29 (245 refs).
23. World Health Organization. Purification of M. leprae. In: Report of the Fifth Meeting of the Scientific Working Group on the Immunology of Leprosy (IMMLEP), 1980. TDR/IMMLEP-SWG(5)/80.3 Annex.
24. Wu, Q.-X., Ye, G.-Y., and Li, X.-Y. Serological activity of natural disaccharide octyl bovine serum albumin (ND-O-BSA) in sera from patients with leprosy, tuberculosis and normal controls. Int. J. Lepr. 56(1987)50-55.
25. Young, D. B., Dissanayake, S., Miller, R. A., Khanolkar, S. R. and Buchanan, T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149(1984)870-873.
1. Ph.D.; Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan.
2. M.Sc; Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan.
3. M.Sc, Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan.
4. M.B.B.S., Department of Microbiology, The Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 74800, Pakistan.
5. M.B.B.S., Marie Adelaide Leprosy Centre, Karachi, Pakistan. H.
6. Ph.D., Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, London, U.K.
7. M.B.B.S., Department of Pathology, University College, London, U.K.
Received for publication on 31 October 1989.
Accepted for publication in revised form on 27 March 1990.