- Volume 58 , Number 3
- Page: 503–11
Sequential monitoring of leprosy patients with serum antibody levels to phenolic glycopipid-I, a synthetic analog of phenolic glycolipid-I, and mycobacterial lipoarabinomannan
ABSTRACT
Sequential serum samples f rom leprosy patients at various stages of antibacterial treatment were tested by an ELISA for antibodies to phenolic glycolipid I (PGL-I), a synthetic PGL-I analog (ND-BSA), and li-poarabinomannan (LAM) f rom Mycobacterium tuberculosis to determine if these antibodies could be useful in monitoring response to therapy. Among patients with positive initial anti-PGL-I IgM, a significant decrease in this antibody was seen over time (p < 0.01), whether assayed by PGL-I or ND-BSA. The two antigens showed good agreement in the detection of decrease in anti-PGL-I IgM. The greatest decrease was seen in patients with a high initial anti-PGL-I IgM and a high bacterial index (BI). Patients with a declining BI were seen to have generally declining antibody levels to PGL-I and to LAM; in those patients with a fluctuating BI, antibody levels were less predictable. We conclude that antibodies to PGL-I and LAM can be useful in following response to therapy in leprosy patients and that cither the native PGL-I or ND-BSA can serve as antigen for the ELISA.RÉSUMÉ
Des échantillons sériques successifs de patients lépreux à différentes étapes du traitement antibactérien furent testés par un ELISA pour la présence d'anticorps vis-à-vis du glycolipide phénolique I (GPL-I), un analogue synthétique du GPL-I (ND-BSA), et un lipoar-abinomannan (LAM) de Mycobacterium tuberculosis pour déterminer si ces anticorps pourraient être utiles dans la surveillance de la réponse au traitement. Parmi les patients positifs au départ pour la présence d'IgM anti-GPL-I, une diminution significative de cet anticorps fut observée au cours du temps (p < 0,01), que le titrage soit fait par GPL-I ou ND-BSA. Les deux antigènes montraient une bonne concordance dans la détection de la diminution des IgM anti-GPL-I. La diminution la plus importante fut observée chez des patients avec des IgM anti-GPL-I et un indice bactériologique (IB) élevés à l'origine. Les patients avec un indice bactériologique en diminution avaient généralement des taux décroissants d'anticorps vis-à-vis de GPL-I et LAM; parmi les patients avec des IB variables, les taux d'anticorps étaient moins prévisibles. Nous concluons que les anticorps vis-à-vis de GPL-I et LAM peuvent être utiles pour suivre la réponse au traitement des malades de la lèpre, et que soit le GPL-I naturel ou le ND-BSA peuvent servir d'antigène pour l'ELISA.RESUMEN
Usando la técnica de ELISA se probaron muestras sccuencialcs de sueros de pacientes con lepra con diferentes tiempos de tratamiento antibacteriano, para buscar anticuerpos contra el glicolípido fenólico I (PGL-I), contra un análogo sintético del PGL-I (ND-BSA), y contra lipoarabinomanana (LAM) de Mycobacterium tuberculosis para determinar si estos anticuerpos podrían ser útiles en el seguimiento de la respuesta a la terapia. Entre los pacientes positivos al inicio para IgM anti-PGL-I, se observó una disminución significativa en los niveles de este anticuerpo proporcional al tiempo del tratamiento (p < 0.01), tanto con el PGL-I como con el ND-BSA. La mayor diminución ocurrió en pacientes con elevados niveles iniciales de IgM anti-PGL-I y con altos índices bacteriológicos (BI). Los pacientes con un BI decreciente también mostraron niveles decrecientes de anticuerpos anti-PGL-I y anti-LAM; en aquellos pacientes con un BI fluctuante, los niveles de anticuerpos fueron menos predeciblcs. Concluímos que los anticuerpos anti-PGL-I y anti-LAM pueden ser útiles en el seguimiento de la respuesta a la terapia en los pacientes con lepra y que tanto el PGL-I nativo como el ND-BSA pueden usarse como antí-geno en la técnica de ELISA.Testing for antibodies to the Mycobacterium leprae-specific phenolic glycolipid-I (PGL-I) antigen has been suggested as a possible tool for detection of new cases of leprosy (1,4,6,21,25,32,33) and for monitoring leprosy patients under treatment (4,8,19,22,29,32). We have previously described a significant correlation between IgM antibodies to PGL-I and the bacterial index (131) (19,30), indicating that anti-PGL-I IgM reflects the total bacillary load of the leprosy patient and, thus, might be of value in following patient response to antibacterial therapy and possible future immunotherapy (24). In this study, to evaluate the usefulness of antibodies to PGL-I in following response to therapy, anti-PGL-I IgM and IgG were measured sequentially in patients under treatment in the New York Regional Hansen's Disease Program. Because PGL-I is obtained from armadillo-derived material, supplies of it are limited. In addition, the water insoluble PGL-I is not easily used in aqueous solutions. In order to resolve these problems, in recent years several groups have produced synthetic PGL-I analogs, with one or more of the antigenic sugar residues of PGL-I covalently attached to protein carrier molecules (3,5). These reports indicate good correlation between native and synthetic PGL-I ELISAs. In order to determine the suitability of synthetic PGL-I for patient monitoring, we have tested one of these glyconjugates, ND-BSA (5), in ELISA for sequential determination of antibodies to PGL-I and compared the performance of ND-BSA to that of native PGL-I. Leprosy patients have been shown to develop high titers of antibodies to mycobacterial arabinomannan antigens. IgG to arabinomannan from M. smegmatis was found to correlate with the BI in leprosy patients and the titer was seen to decrease during treatment (23). Lipoarabinomannan (LAM) from M. leprae and M. tuberculosis have been purified and partially characterized structurally and immunologically, with serological crossreactivity seen between the LAMs of the two different bacteria (12). In leprosy patients, a correlation has been seen between the BI and both IgG and IgM to M. tuberculosis LAM (18), suggesting that anti-LAM antibodies might also be useful for following leprosy patients' responses to therapy.
To determine to what extent changes in antibodies to PGL-I and LAM reflect changes in the bacillary load, sequential anti-PGL-I and LAM antibodies were quantitated in patients who had also been tested for sequential BI. Results indicate that in most patients with a steadily declining BI, a decrease in the BI was accompanied by a decrease in antibodies to PGL-I and/or LAM. ND-BSA and native glycolipid both appeared to be suitable for detection of sequential antibodies to PGL-I.
MATERIALS AND METHODS
Patients. Antibody determinations were performed on patients at various stages of treatment at the New York Regional Hansen's Disease Program. The treatment regimen for individual patients depended upon the stage of treatment and disease activity. Patients showing bacillary activity were treated with standard multidrug therapy consisting of dapsone and rifampin, and in cases of chronic or recalcitrant reaction, clofazimine. Most inactive patients were maintained on dapsone monotherapy. Thalidomide and/or prednisone were used for treatment of reactions (13,17). Patients were classified clinically and histopathologically according to the Ridley-Jopling scale (27). The BI was measured according to the method of Ridley (26). The BI and histo-pathology were determined on punch biopsies at the Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A. Sera were collected by venipuncture and stored in aliquots at - 70ºC until used. Previous studies (16,18,28) had indicated patients that were positive for antibodies to PGL-I and/ or LAM. Three groups of patients were used for analyses in this report. The first group, consisting of 47 patients, was used to test for sequential detection of anti-PGL-I incorporated into liposomes. A second group of 22 patients was used to compare detection of specific IgM by native PGL-I liposomes and the synthetic PGL-I analog ND-BSA. A third group of 24 patients was chosen to study the relationship between sequential BI and sequential antibodies to PGL-I and LAM. The criteria for inclusion in this group were: a) A total length of time of at least 24 months between the first and last serum studies for each individual; b) A BI determination had to have been done within ±2 months of each serum sample assayed.
Antigens for ELISA. PGL-I, LAM, and ND-BSA were provided under a National Institutes of Health contract by Dr. P. J. Brennan (Colorado State University, Fort Collins, Colorado, U.S.A.). PGL-I was purified from armadillo-derived material as previously described (11). The synthesis of several of the synthetic PGL-I glycoconju-gates has been reported (5,9). The protocol for the purification of LAM (called LAM-B in the original reference) from M. tuberculosis and M. leprae was previously described (12).
ELISA for antibodies to PGL-I using PGL-I liposomes. Antibodies to the PGL-I antigen of M. leprae were detected by an enzyme-linked immunosorbent assay as previously described (19,28). Briefly, PGL-I was incorporated into liposomes with sphingomyelin, cholesterol, and dicetyl phosphate. Control liposomes were made without PGL-I. PGL-I and control liposome suspensions were coated onto polystyrene microtiter plates (Dynatech Laboratories, Inc., Alexandria, Virginia, U.S.A.). After the plates were coated, they were washed three times with phosphate buffered saline (PBS) and then blocked with PBS plus 3% bovine scrum albumin (PBS-BSA). Sera diluted in PBS-BSA were added in duplicate to wells containing PGL-I liposomes and control liposomes and incubated at 37ºC. Plates were then washed three times with PBS, and goat anti-human IgM (or IgG) peroxidase conjugate (Cooper Biomedical, Inc., West Chester, Pennsylvania, U.S.A.) diluted 1:1000 in PBS-BSA was added. Plates were washed again, and the substrate solution was added (1.8 mM 2, 2'azino-di[3-ethylbenzthiazolin-6-sulfonic acid] (Boehringer Mannheim Biochemicals, Indianapolis, Indiana, U.S.A.) -0.1 mM H2O2 in phosphate buffer) for 1 hr at room temperature. The reaction was stopped with 0.32% NaF and extinction (E) was read at 405 nm. Results were expressed as ΔE = E (PGL-I liposome coat) - E (control liposome coat). Initial antibody readings to PGL-I were designated as ΔEi and final readings as ΔEf. A ΔE > 0.10 at serum dilution of 1/20 was considered a positive reading for anti-PGL-I IgM and IgG (20). Patients that were positive by preliminary screening for ΔE anti-PGL-I IgM and/or IgG were tested sequentially for both isotypes. Patients exhibiting either a positive ΔEi or ΔEf for a given isotype were used for statistical analysis of that isotype. Initial and final sera for each individual were assayed simultaneously on the same microtiter plate. Previous experience (19,28) showed that, with a serum dilution of 1/20, the above protocol was effective for detection of anti-PGL-I IgM and IgG in most patients. However, a number of patients were found to have very high levels of anti-PGL-I IgM, such that, with sera diluted 1/20 the enzymatic reaction would plateau well before the 1-hr substrate incubation period. In order to prevent changes in antibody level from being obscured in this way, high anti-PGL-I IgM patients' sera were diluted such that serial dilutions yielded proportional AE values. These values were then corrected to 1/20 by multiplying by the dilution factor (i.e., x 4 for sera tested at 1/80). In order to determine whether sequential anti-PGL-I IgM levels were significantly different, regression analysis was performed on duplicate scrum samples from 37 patients whose anti-PGL-I IgM levels ranged from negative to very high. Pearson's correlation coefficient between duplicate serum samples was calculated (r = 0.98). A figure of two times the standard deviation of the differences in the means was used to determine significant differences (ΔE = 0.16, p < 0.03).
ELISA for antibodies to PGL-I using ND-BSA. The ELISA using ND-BSA was similar to ELISA with PGL-I liposomes with the following exceptions: a) For coating plates, ND-BSA was dissolved in carbonate-bicarbonate buffer, pH 9.6, at 0.5 μg carbohydrate/ml. Control wells were coated with BSA at a concentration identical to that in the ND-BSA-coated wells, b) Blocking and diluent for sera and antibody-enzyme conjugate was PBS + 1% BSA + 0.05% Tween 20.
ELISA for antibodies to LAM. The ELISA for antibodies to LAM was identical to the ELISA using PGL-I liposomes with the following exceptions: a) LAM antigen was coated onto microtiter plates at 2 μg/ ml in carbonate-bicarbonate buffer, pH 9.6. Control wells were treated with coating buffer alone, b) Sera were diluted routinely 1/200, 1/100, and 1/20 for detection of anti-LAM IgG, IgM and IgA, respectively. For sequential detection of anti-LAM antibodies in patients with very high levels, higher dilutions of serum were used, c) Substrate buffer was citrate-phosphate buffer, pH 5, with a 20-min substrate incubation period, d) Cut-off points for seropositivity were established as ΔE = 0.27, 0.14, and 0.19 for anti-LAM IgG, IgM, and IgA, respectively. These values represent the mean + 2 S.D. of 35 control sera (18).
Statistical analysis. The initial and final antibody values were compared by the non-parametric Wilcoxon signed rank test. Pearson's correlation coefficient was calculated to examine the relationship between the BI and the rate of decrease of anti-PGL-I IgM. Correlation coefficients were also calculated to compare detection of antibodies by PGL-I liposomes and ND-BSA.
RESULTS
Sequential analysis of serum antibodies indicate that anti-PGL-I-positive leprosy patients experienced a drop in the level of anti-PGL-I IgM over time. However, patients positive for anti-PGL-I IgG did not exhibit a significant drop in anti-PGL-I IgG over an almost identical mean time span (Table 1). The final reading for anti-PGL-I IgM was significantly lower than the initial reading in the group of 47 patients tested by PGL-I liposome ELISA. The group of patients most responsible for the drop in anti-PGL-I IgM were those with a high AEi anti-PGL-I IgM (Table 1). These patients had a significant decrease in anti-PGL-I IgM, while those with a low AEi anti-PGL-I IgM did not. While the former group had a slightly longer mean time between ΔEi and ΔEf than did the latter, it was not significantly longer and, thus, probably did not contribute to the differences seen between these two groups. Additionally, the rate of decrease of anti-PGL-I IgM was found to correlate with the BI, indicating a faster drop-off in anti-PGL-I IgM levels in high BI patients (Fig. 1).
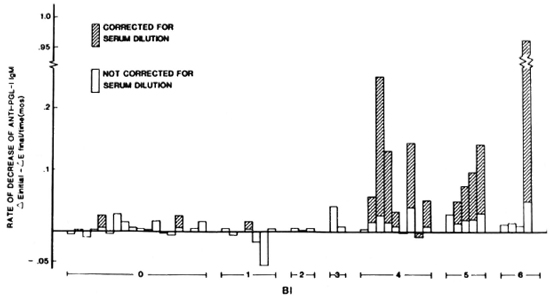
Fig. 1. Rate of decrease of anti-PGL-I IgM in patients grouped by bacterial index (BI). Pearson's correlation coefficient for rate of decrease (corrected for serum dilution) versus BI: r = 0.4040, p < 0.01.
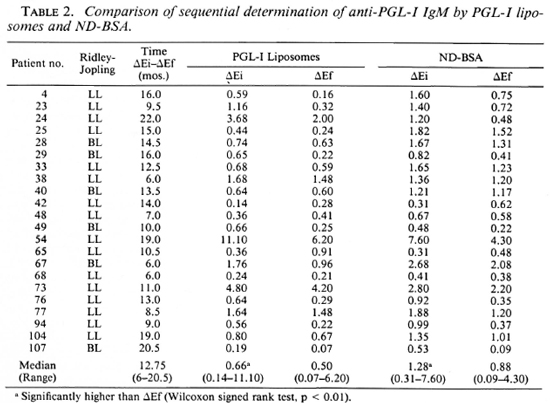
ND-BSA gave similar results to PGL-I liposomes in sequential analysis of anti-PGL-I IgM (Table 2). In testing of identical scrum samples, both ELISA systems showed significantly lower ΔEf anti-PGL-I IgM. There was good correlation between the two assays in the detection of anti-PGL-I IgM for ΔEi (r = 0.9203, p < 0.001) and ΔEf (r = 0.8454, p < 0.001). It should be noted that only 2 of 22 patients (nos. 42 and 65) surveyed by both PGL-I liposomes and ND-BSA showed a marked increase in anti-PGL-I IgM, and that both assays detected this increase. Twelve of the 22 patients shown in Table 2 decreased significantly in anti-PGL-I IgM as measured by PGL-I liposomes (decrease in ΔE > 0.16); 2 increased significantly, 7 showed decreases that were not significant, and 1 had an increase that was not significant.
The results of sequential determination of antibodies and BI arc presented in Figures 2 and 3. Sequential antibody levels are shown only for those antibodies that exhibited at least one positive reading. Figures 2 and 3 show 12 patients with an initial BI of > 3 + . It can be seen that in patients with a consistently declining BI (Fig. 2 A, B, C, D, E; Fig. 3 A, E, F) most also experienced a decline in antibody levels (Fig. 2 A, B, C, D; Fig. 3 E, F). Thus, among patients with a consistently declining BI, 6 of 8 showed declining antibody levels, and 1 (Fig. 2E) showed a decline in three of four antibodies. Among patients with an initial BI of > 3 + and a BI that did not drop consistently (Fig. 2F; Fig. 3 B, C, D) antibody patterns were less predictable.
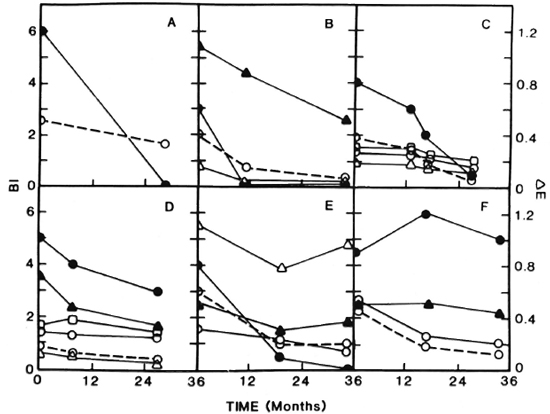
Fig. 2. Sequential determination of bacterial index (BI) and antibodies to PGL-I and LAM in patients with an initial BI of > 3 +. 

 = BI;
= BI;  ---
--- = anti-PGL-I IgM; Δ
= anti-PGL-I IgM; Δ Δ = anti-PGL-I IgG;
Δ = anti-PGL-I IgG; 

 = anti-LAM IgM; ▲
= anti-LAM IgM; ▲ ▲ = anti-LAM IgG;
▲ = anti-LAM IgG;  ---
--- = anti-LAM IgA.
= anti-LAM IgA.
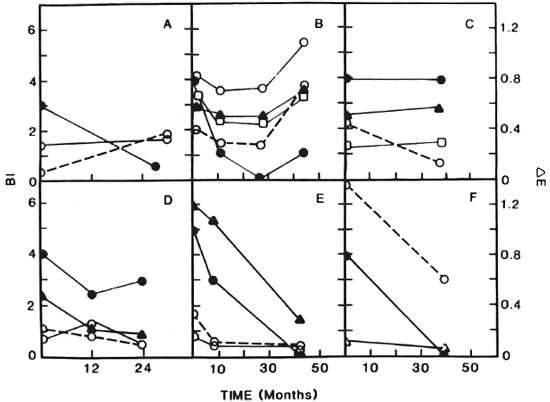
Fig. 3. Sequential determination of BI and antibodies to PGL-I and LAM in patients with a BI of >3 +.
In addition to the 12 patients depicted in Figures 2 and 3, 12 additional patients were tested for sequential antibodies and BI. Three of these were negative for both the BI and antibodies throughout the study. Four had Bis that decreased consistently, with 3 of the 4 showing a consistent decrease in antibodies; the remaining 5 had Bis that did not decrease steadily and, as with such patients depicted in Figures 2 and 3, their antibody patterns were unpredictable (data not shown). Thus, overall, in this study we have seen that among patients with a steadily declining BI, 75% also showed steadily declining antibody levels. In patient B, Figure 3, the decline and then rise in BI was reflected by increases in antibody levels. The patient, who had dapsone-resistant disease by mouse foot-pad testing and elevated liver function tests, received clofazimine and dapsone. Eight months before the rise in the BI and antibodies was seen, dapsone was decreased from 100 to 50 mg daily because of mild renal insufficiency. During the course of the study there was evidence of disease progression. Specifically, the patient experienced impotence with elevated FSH and LH (data not shown) which was successfully treated with 200 mg twice monthly of dela-testryl (Squibb, Princeton, New Jersey, U.S.A.). Disease progression in this patient was also evidenced by a deterioration in nerve function as measured by nerve conduction testing (data not shown).
DISCUSSION
This study indicates that monitoring of leprosy patients' responses to therapy with antibodies to PGL-I and LAM provides a valuable adjunct to skin biopsy and slit-skin smears. The largest and most rapid decreases in anti-PGL-I IgM were found to occur in patients with a high initial anti-PGL-I IgM and a high BI. This may reflect clearance of large numbers of bacilli in patients early in treatment. The tendency for low anti-PGL-I IgM patients to remain seropositive may be related to bacillary persistence, a known phenomenon of leprosy (31). Alternatively, the PGL-I antigen itself is not water soluble and may remain in tissues for long periods of time, stimulating a low-level antibody response in the absence of viable bacilli.
IgG to PGL-I did not drop in the group of patients who were initially positive for this antibody. Similarly, Bach, et al. (2) found that IgG to whole M. leprae decreased more slowly than IgM to whole M. leprae and PGL-I in lepromatous patients. Douglas, et al. (8) also found a decrease in antibodies to both PGL-I and a synthetic PGL-I glycon-jugate in leprosy patients under treatment, although this study did not distinguish between the immunoglobulin class of the anti-PGL-I response. So far, studies in humans suggest that anti-PGL-I IgG is indicative of upgrading or an intact T-cell response (16,19). Factors regulating IgG production arc being studied intensely in a variety of systems. IgG production seems to depend on a variety of soluble T-cell products in mice and probably also in humans (10). In leprosy, evidence exists for the deficiency of T-cell products (14), and factors involved in the isotype switch mechanism may be a part of the various forms of disease seen throughout the leprosy spectrum.
IgM is a classical neoantigenic response. Therefore, IgM persistence may be indicative of antigenic persistence. Findings similar to those herein reported with leprosy have been noted in Lyme disease (7), where persistent specific IgM is indicative of prolonged and severe disease, and in hepatitis B infection, where IgM levels to hepatitis B core antigen correlated with disease activity (15). Currently, prevalent clinical practice for monitoring multibacillary leprosy involves detection of bacilli in the skin. Our findings show some correlation with serum antibodies to PGL-I and LAM and the prevalence of bacilli in the skin. However, a significant percentage of patients who are negative by skin bacillary criteria still show elevated levels of antibody to PGL-I and/or LAM. Further studies are needed before it can be determined whether an individual's antibody levels have stabilized, or whether increasing or persistently elevated antibody readings are indicative of inadequate therapy or relapse.
Acknowledgment. This work was sponsored by the National Hansen's Disease Program of the U.S. Department of Health and Human Services.
REFERENCES
1. Agis, F., Schlich, P., Cartel, J.-L., Guidi, C. and Bach, M.-A. Use of anti-M. leprae phenolic gly-colipid-I antibody detection for early diagnosis and prognosis of leprosy. Int. J. Lepr. 56(1988)527-536.
2. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
3. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugatcs containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
4. Britton, W. J., Garsia, R. J. and Basten, A. The serological response to the phenolic glycolipid of Mycobacterium leprae in Australian and Nepali patients. Aust. N.Z. J. Med. 17(1987)568-573.
5. Cho, S.-N., Hunter, S. W., Rea, T. H., Gelder, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-β-d-glu-copyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
6. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
7. Craft, J. E., Fischer, D. K., Shimamoto, G. T. and Steere, A. C. Antigens of Borrelia burgdorfer, recognized during Lyme disease. J. Clin. Invest. 7(1986)934-939.
8. Douglas, J. T., Steven, L. M., Fajardo, T., Cellona, R. V., Madarang, M. G. and Steen-bergen, G. J. The effects of chemotherapy on antibody levels in lepromatous leprosy. Lepr. Rev. 59(1988)127-135.
9. Fujiwara, T., Hunter, S. W„ Cho, S.-N., Aspi-nall, G. O. and Brennan, P. J. Chemical synthesis and serology of the disaccharides and trisaccharides of phenolic glycolipid antigens from leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy, infect. Immun. 43(1984)245-252.
10. Howard, M. and Paul, W. E. Regulation of B-cell growth and differentiation by soluble factors. Ann. Rev. Immunol. 1(1983)307-333.
11. Hunter, S. W., Fujiwara, T. and Brennan, P. J. Structure and antigenicity of the major specific glycolipid antigen oí Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
12. Hunter, S. W., Gaylord, H. and Brennan, P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 261(1986)12345-12351.
13. Jacobson, R. R. The treatment of leprosy (Hansen's disease). Hosp. Form. 17(1982)1076-1091.
14. Kaplan, G. and Cohn, Z. A. The immunobiology of leprosy. Int. Rev. Exp. Pathol. 28(1986) 45-78.
15. Koike, K., Iino, S., Kurai, K., Mitamura, K., Endo, Y. and Oka, H. IgM anti-HBc in anti-HBe positive chronic type B hepatitis with acute exacerbations. Hepatology 7 (1987) 573-576.
16. Koster, F. T., Scollard, D. M., Umland, E. T., Fishbein, D. B., Hanly, W. C, Brennan, P. J. and Nelson, K. E. Cellular and humoral immune response to a phenolic glycolipid antigen (PhenGL-I) in patients with leprosy. J. Clin. Microbiol. 25(1987)551-556.
17. Levis, W. R. Treatment of leprosy in the United States. Bull. N.Y. Acad. Med. 60(1984)696-711.
18. Levis, W. R., Meeker, H. C, Schuller-Levis, G., Sersen, E., Brennan, P. J. and Fried, P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J. Infect. Dis. 156(1987)763-769.
19. Levis, W. R., Meeker, H. C, Schuller-Levis, G., Sersen, E. and Schwerer, B. IgM and IgG antibodies to phenolic glycolipid I from Mycobacterium leprae in leprosy: insight into patient monitoring, erythema nodosum leprosum and bacillary persistence. J. Invest. Dermatol. 86(1986)529-534.
20. Meeker, H. C, Levis, W. R., Sersen, E., Schuller-Levis, G., Brennan, P. J. and Buchanan, T. M. ELISA detection of IgM antibodies against phenolic glycolipid-I in the management of leprosy: a comprison between laboratories. Int. J. Lepr. 54(1986)530-539.
21. Menzel, S., Harboe, M., Bergsvik, H. and Brennan, P. J. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy household contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
22. Miller, R. A., Gorder, D. and Harnisch, J. P. Antibodies to phenolic glycolipid-I during long-term therapy: serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
23. Miller, R. A., Harnisch, J. P. and Buchanan, T. M. Antibodies to mycobacterial arabinoman-nan in leprosy: correlation with reactional states and variation during treatment. Int. J. Lepr. 52(1984)133-139.
24. Nathan, C. F., Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systemic effects of intradermal recombinant inlcrferon-gamma in patients with lep-romatous leprosy. N. Engl. J. Med. 315(1986)6-15.
25. Petchclai, B., Khupulsup, K.., Hiranras, S., Sampattanovich, S., Sampoonachot, P. and Leelarusamee, A. A passive hemagglutination test for leprosy using a synthetic disaccharide antigen. Int. J. Lepr. 56(1988)255-258.
26. Ridley, D. S., Appendix III. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G., and Davey, T.F., Eds. Baltimore: The Williams and Wilkins Company, 1964, pp. 620-622.
27. Ridley, D. S. and Joplino, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-279.
28. Schwerer, B., Meeker, H. C, Sersen, E. and Levis, W. R. IgM antibodies against phenolic gly-colipid I from Mycobacterium leprae in leprosy sera: relationship to bacterial index and erythema nodosum leprosum. Acta Leprol. 2(1984)395402.
29. Sinha, S., McEntegart, A., Girdhar, B. K., Bhatia, A. S. and Sengupta, U. Appraisal of two Mycobacterium leprac-spccihc serological assays for monitoring chemotherapy in lepromatous(LL/ BL) leprosy patients. Int. J. Lepr. 57(1989)24-32.
30. Waters, M., Rees, R. and McDougall, A. Ten years of dapsone in lepromatous leprosy: clinical bacteriological, and histological assessment and the finding of viable leprosy bacilli. Lepr. Rev. 45(1978)288-298.
31. Wemambu, S. N. C, Turk, J. L., Waters, M. F. R. and Rees, R. J. W. Erythema nodosum leprosum: a clinical manifestation of the Arthus phenomenon. Lancet 2(1969)933-935.
32. Wu, Q.-X., Ye, G.-Y. and Li, X.-Y. Serological activity of natural disaccharide octyl bovine serum albumin (ND-O-BSA) in sera from patients with leprosy, tuberculosis and normal controls. Int. J. Lepr. 56(1988)50-55.
33. Young, D. B. and Buchanan, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(2983)1057-1059.
1. M.S.; New York State Institute for Basic Research in Developmental Disabilities, 1050 Forest Hill Road, Staten Island, New York 10314.
2. Ph.D., New York State Institute for Basic Research in Developmental Disabilities, 1050 Forest Hill Road, Staten Island, New York 10314.
3. Ph.D., New York State Institute for Basic Research in Developmental Disabilities, 1050 Forest Hill Road, Staten Island, New York 10314.
4. M.D.; Department of Dermatology, New York Medical College, Bayley Seton Hospital, Staten Island, New York 10304, U.S.A.
5. M.D.; Department of Dermatology, New York Medical College, Bayley Seton Hospital, Staten Island, New York 10304, U.S.A.
6. M. D., Department of Dermatology, New York Medical College, Bayley Seton Hospital, Staten Island, New York 10304, U.S.A.
Reprint requests to Dr. William R. Levis.
Received for publication on 27 December 1988.
Accepted for publication in revised form on 19 March 1990.