- Volume 58 , Number 3
- Page: 512–7
Assessment of anti-phenolic glycolipid-I IgM levels using an ELISA for detection of M. leprae infection in populations of the South Pacific Islands
ABSTRACT
Anti-phenolic glycolipid-I (PGL-I) IgM levels were determined in 96% of the general population of the Southern Marquesas and Maupiti, remote islands of French Polynesia, where the average annual detection rates of leprosy during the past 30 years have been 57.1 and 4.4 per 100,000, respectively. The seropositivity in these two areas was 4.3% and 4.2%, respectively. No significant difference (p > 0.05) was found between cither these two figures or between the percentages of persons with high (> 0.500 OD) anti-PGL-I IgM levels (9.2% and 5.3%). In the two islands, the age distributions of anti-PGL-I IgM were very similar; the percentage of positive responders was higher in females than in males and higher in adolescents than in adults. These results suggest that the usefulness of the determination of anti-PGL-I IgM levels by ELISA, using the synthetic trisaccharide as antigen, for detecting Mycobacterium leprae infection in leprosy control programs is extremely doubtful.RÉSUMÉ
Les taux d'IgM anti-glycolipide phénolique I (GPL-I) ont été déterminés parmi 96% de la population générale des Marquises Australes et Maupiti, îles éloignées de la Polynésie Française, où les taux annuels moyens de détection durant les 30 dernières années ont été respectivement de 57,1 et 4,4 pour 100.000. La séropositivité dans ces deux régions était respectivement de 4,3% et 4,2%. Aucune différence significative (p > 0,05) ne fut trouvée ni entre ces taux, ni entre les pourcentages de personnes présentant des taux élevés (>0,500 OD) d'IgM anti-GPL-I (9,2% et 5,3%). Dans les deux îles, la distribution par âge des anticorps anti-GPL-I étaient très semblables; le pourcentage de positifs était plus élevé chez les femmes que chez les hommes, et chez les adolescents que chez les adultes. Ces résultats suggèrent que l'utilité, pour la détection de l'infection par Mycobacterium leprae dans les programmes de lutte contre la lèpre, de la détermination des taux d'IgM anti-GPL-I par ELISA en utilisant le trisaccharide synthétique comme antigène, est très incertaine.RESUMEN
Se determinaron los niveles de IgM anti-glicolípido fenólico-I (PGL-I) en el 96% de la población general de las Marquesas del Sur y de Maupili, remotas islas de la Polinesia Francesa, donde la incidencia de lepra en los últimos 30 años ha sido, respectivamente, de 57.1 y de 4.4 por 100,000. La seropositividad en estas 2 áreas fue del 4.3% y del 4.2%, respectivamente. No hubo una diferencia estadísticamente significativa entre estas dos cifras (p > 0.05) ni entre los porcentajes de personas con altos niveles (D.O. > 0.5) de IgM anti-PGL-I (9.2% y 5.3%). Las distribuciones por edades de IgM anti-PGL-I fueron muy similares en las dos islas; los porcentajes de positividad fueron mayores en mujeres que en hombres y mayores en adolescentes que en adultos. Estos resultados sugieren que la utilidad de la determinación de los niveles de IgM anti-PGL-I por ELISA (usando como antígeno el trisacárido sintético) para detectar la infección por Mycobacterium leprae en los programas de control contra la lepra, es extremadamente dudosa.Investigation of subclinical Mycobacterium leprae infection using immunological tools has been recognized as one of the priority areas of epidemiological research (17). During the past decade, serological tests for the detection of anti-M. leprae antibodies have been developed. Among them, the enzyme-linked immunosorbent assay (ELISA) using phenolic glycolipid-I (PGL-I) as a M. leprae-specific antigen has proved to be potentially useful for the serological study of leprosy patients, contacts, and "normal" individuals (4). Further studies suggested it might be appropriate for large-scale serological trials (1,2,9,14). In the Southern Marquesas, on the implementation of a program of chemoprophylaxis of leprosy (8), blood samples were collected from 96% of the population for the determination of anti-PGL-I IgM by ELISA. The results of this serological investigation showed that there was a comparable age distribution between the anti-PGL-I IgM-pos-itive responders and the leprosy cases, suggesting that a relation might exist between detection rates of leprosy and anti-PGL-I IgM prevalence rates in a population. These findings and the fact that, in French Polynesia, detection rates of leprosy vary greatly from island to island (7) prompted us to undertake a similar investigation in another place where the leprosy detection rate was different in order to determine whether or not the age distribution of anti-PGL-I IgM was also different. The aim of this paper is to report and to analyze the results of both investigations.
MATERIALS AND METHODS
The first investigation was carried out in the Southern Marquesas islands in 1988; the second, in Maupiti island in 1989. During the last 30 years (1958-1987), 37 new leprosy patients- 15 females (9 paucibacillary and 6 multibacillary) and 22 males (14 paucibacillary and 8 multibacillary) -were detected in the Southern Marquesas, giving an average leprosy detection rate of 57.1 per 100,000. During the same period (19581987), the diagnosis of leprosy (LL) was made in only one man in 1970 in Maupiti, giving an average detection rate of 4.4 per 100,000.
In the two islands, blood samples were obtained by the finger-prick method and dropped on Whatman no. 1 filter-paper cards (L.D.A., Saint Brieuc, France) on which were written identification data on the persons from whom the blood was collected. From each person, eight spots were collected, air dried, stored in plastic bags, and sent to the laboratory of the Malarde Institute in Papeete, Tahiti. As in previous studies (10), the anti-PGL-I IgM levels were determined by using the natural trisaccha-ride-3-p-hydroxy-phenylpropionate-bo-vine serum albumin (NTP) as antigen and a cut-off level of 0.200 optical density (OD) (calculated as the mean ± 2 standard deviations of OD values determined from "healthy" Polynesian subjects) was chosen (11). The filter-paper disc was detached from the card, eluted into 500 μlof phosphate-buffered saline, pH 7.2, plus 0.1% Tween 20 and 5% bovine serum albumin to an equivalent dilution of 1/250. The OD was read at 492 nm using a microplate ELISA reader (Titertek, Helsinki, Finland) connected to an IBM-PC-compatible microcomputer. A clinical examination, the search for acid-fast bacilli (AFB) in slit-skin smears as well as a measure of PGL-I antigen level in sera collected by venipuncture, according to the method described by Cho, et al. (12,13), were performed for all of the persons with an anti-PGL-I IgM level > 0.200.
RESULTS
The blood samples were collected in 3 weeks from 2677 (96.1%) of the 2868 Southern Marquesas inhabitants and in 2 weeks from 890 (95.7%) of the 930 Maupiti inhabitants (Table 1).

In the Southern Marquesas in 1988, 119 (4.4%) of the 2677 collected sera were anti-PGL-I IgM "positive" (Table 1). Of these, 67 (56.3%) had an OD ranging from 0.200 to 0.300, 41 (34.5%) had an OD ranging from 0.300 to 0.500, and 11 (9.2%) had an OD > 0.500 which is the lowest level we have observed in untreated multibacillary patients in French Polynesia (11). No signs of leprosy were found in the 119 persons, and the searches for AFB in slit-skin smears as well as PGL-I antigen determinations were also negative. The age distribution (for patients, age of diagnosis) of both the anti-PGL-I IgM-positive persons of the Southern Marquesas and the 37 cases of leprosy detected during the 1958-1987 period are presented in Figure 1. The number of persons with IgM-positive serum increases rapidly after infancy, reaching a maximum peak in the 10-20-year-old age group, then decreasing steadily to zero by 60 years of age. Among the leprosy patients, the number of cases increases after childhood, reaching a peak of 19 cases in the 20-30-year-old age group and then decreasing steadily to zero by 60 years of age. Although the overall aspects of the two curves have much similarity, the peak of leprosy cases occurred at an older age than that of the IgM-positive healthy persons. It is tempting to consider that the difference between the two peaks probably reflects the incubation period of clinical leprosy which is assumed to be several years after infection by M. leprae (16).
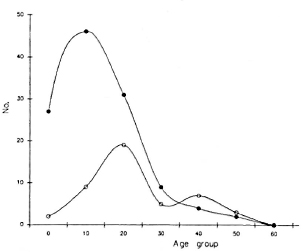
Fig. 1. Anti-PGL-I IgM-positive persons (- -) and leprosy patients (-
-) and leprosy patients (- -) age-related numbers in the Southern Marquesas.
-) age-related numbers in the Southern Marquesas.
In 1989, blood samples were collected again from 108 of the 119 positive and from 208 of the negative South Marquesan re-sponders. IgM levels remained stable in 91 (84.3%) of the seropositive samples collected; a decrease was observed in 9 (8.3%), of which 7 became seronegative, and an increase was observed in 8 (7.4%). The ODs of two persons in the last group were more than 1.000, very substantially higher than the levels observed the year before. In the 208 seronegative persons, 5 (2.4%) were found seropositive with ODs of 0.2000.300. In 1989, we reexamined all of these positive responders. No signs of leprosy were found, and slit-skin smears as well as the PGL-I antigen assays remained negative.
In Maupiti island, 38 (4.3%) of the 890 collected sera were positive (Table 1); 20 (52.6%) had ODs ranging from 0.200 to 0.300, 16 (42%) had ODs ranging from 0.300 to 0.500, and 2 (5.3%) had ODs > 0.500. Among the 38 persons with a positive scrum, no signs of leprosy were found and slit-skin smears and PGL-I antigen determinations were negative.
The age-related distribution curves of anti-PGL-I IgM prevalence rates among the positive responders arc very much similar between the Southern Marquesas and the Maupiti islands (Fig. 2). In both places, the prevalence rate of IgM-positive persons increased rapidly after infancy, reaching a peak of 8% in the 10-20-year-old age group in the Maupitians and in the 20-30-year-old age group in the Marquesans. Then, a steep decrease is observed, followed by a slight peak in older persons (over 40 years of age in the Marquesas, over 50 years of age in Maupiti).
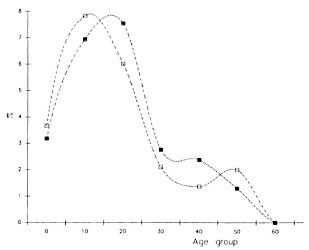
Fig. 2. Anti-PGL-I IgM age-specific prevalence rates in the Southern Marquesas (- -) and Maupiti (-
-) and Maupiti (- -) islands.
-) islands.
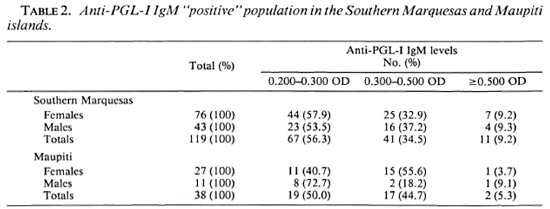
The percentages of positive responders were higher among females than among males in the populations of both islands. In the Southern Marquesas, of the 119 seropositive persons, 64% were females; in Maupiti, of the 38 seropositive persons, 71% were females (Table 2).
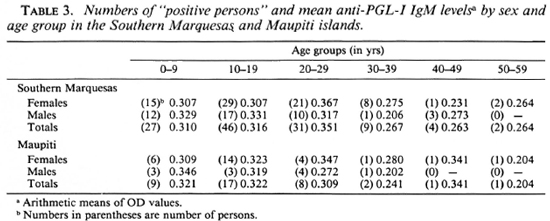
The arithmetic means of anti-PGL-I IgM levels by sex and by age group are presented in Table 3. No significant differences (p > 0.05) were found in the IgM levels by age group or by sex, nor between the positive responders in the populations of each island nor between the positive populations of the two islands.
DISCUSSION
Because long-term follow-up in evaluating the sensitivity and specificity of the predictive value of the ELISA-PGL-I antibody test for detecting M. leprae infection is time consuming and expensive, to compare the ELISA results among areas with various endemicity levels of leprosy seems to be a practical approach.
As usual, the sensitivity of any diagnostic test is linked to its specificity, and a high sensitivity can only be achieved at the expense of a considerable loss in specificity. In the field application of a serodiagnostic test, it is preferable to opt for high specificity (6). The selection of a 0.200 OD as a posi-tivity criterion of the ELISA is based on our previous studies which showed that this criterion resulted in 97.5% specificity among the "noncontact" Polynesian population and in 96% and 37% sensitivity, respectively, in multibacillary and paucibacillary leprosy patients (11).
The Southern Marquesas are a leprosy-endemic area with an average detection rate during the past 30 years of 57.1 per 100,000. The discovery of 119 positive responders, a 4.4% anti-PGL-I antibody-positive rate, among the healthy Southern Marquesan population is not a striking finding. In fact, it was thought that the infection with M. leprae is far more common than the number of overt cases (17). For example, in a population of New Guinea, in which the prevalence rate of leprosy was 9.9%, the prevalence rate of anti-PGL-I IgM was 32% (1,3).
The most surprising and disappointing observations from the current study are that the anti-PGL-I antibody-positive rate (4.3%) and the proportion of high (> 0.500 OD) PGL-I IgM level among the positive responders (5.3%) in the population from Maupiti do not differ significantly (p > 0.05) from that of the Southern Marquesas, despite the fact that leprosy is rare and the average detection rate is only 4.4 per 100,000 in Maupiti. These findings could not be attributed to the fact that determinations of anti-PGL-I antibody were conducted in the Southern Marquesas 15 months earlier than those in Maupiti, because the assays are routinely performed in our laboratory with highly reproducible results at various time intervals (11). In addition, in 1989 we repeated the assays among 119 positive responders and 208 negative responders from the Southern Marquesas who had been tested in 1988, and the results of 1989 were similar to those of 1988 in 84.3% of the positive responders and in 97.6% of the negative responders. Therefore, technical artifacts are unlikely to be responsible for the similarity of the results obtained from the Southern Marquesas and Maupiti. Among other explanations, the differences of socioeconomic status, sanitary conditions, and genetic background between the two populations should be considered. In fact, the differences of these items between the two populations are practically nil (3,5). Fine, et al. also reported similarly disappointing results from a study conducted in Malawi (14). They could not detect a significant difference in the proportion of anti-PGL-I IgM-positive responders between contacts and noncon-tacts.
Although it has been reported that antibodies against At. leprae-derived glycolipid were detected only in sera from leprosy patients, and not in sera from uninfected individuals or patients infected with mycobacteria other than M. leprae (15), our results suggest that the test using synthetic trisac-charide as antigen lacks specificity. It is possible that the positive reactions in our study are irrelevant to the infection of M. leprae. Despite the fact that the terminal trisac-charide immunodominant epitope of the native PGL-I is chemically unique among mycobacteria tested, and antibody response was proved to distinguish between the phenolic glycolipid from M. leprae and the structurally related phenolic glycolipids from M. bovis and M. kansasii (15), it may be speculated that other antigens, chemically different but sterically resembling PGL-I, exist and, thus, induce crossreactivity. The fact that we failed to extract PGL-I antigen from the sera of the positive responders in the current study, even from responders who showed high IgM levels (> 1.000 OD), also supports this hypothesis. Whatever the explanations, the usefulness of measuring the anti-PGL-I IgM level by ELISA, using the synthetic trisaccharide as antigen, for detecting M. leprae infection in leprosy control programs is extremely doubtful.
Acknowledgments. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases; from ILEP organizations (Fondations Raoul Follereau, France, and the Leprosy Trust Board, New Zealand), and from the Commission de Coordination de la Recherche dans les Départements et Territoires d'Outre-Mer (CORDET).
We express our thanks for C. Plichart and P. Lu-quiaud for their technical assistance in the laboratory, and to Mrs. O. Falchetto and Miss V. Chenon for their work in the field. Wc are indebted to Mrs. V. Matuaiti and to Mrs. D. Schlegel of the Public Health Service in the Marquesas islands for their assistance in the field.
REFERENCES
1. Bagshawe, S., de Burgh, S., Fung, S. C, Chuah, J. and Berry, G. The epidemiology of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 83(1989)121-127.
2. Baumgart, K., Britton, W., Basten, A. and Bagshawe, A. Use of phenolic glycolipid I for serodiagnosis of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81(1987)1030-1032.
3. Belwood, P. Les Polynésiens et Leur Culture. Archéologie et Histoire. Papeéte: Ed. du Pacifique, 1983.
4. Buchanan, T., Dissanyake, S., Young, D. B., Miller, R. A., Acedo, J. R., Harnisch, J. P., Khanolkar, S. R. and Estrada-Parra, S. Evaluation of the significance of antibodies to phenolic glycolipid of Mycobacterium leprae in leprosy patients and their contacts. (Abstract) Int. J. Lepr. 51(1983) 658-659.
5. Buck, P. H. Les Migrations des Polynésiens. Paris: Ed. Payot. (Biblio. Sc.), 1952.
6. Burgess, P. J., Fine, P. E. M., Ponnighaus, J. M. and Draper, C. Serological tests in leprosy; the sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidem. Infect. 101(1988)159-171.
7. Cartel, J.-L., Boutin, J.-P., Plichart, R., Roux, J. and Grosset, J.-H. La lèpre dans les archipels de Polynésie Française de 1967 à 1987. Bull. Soc. Pathol. Exot. Filiales 81(1988)819-826.
8. Cartel, J.-L., Chanteau, S., Boutin, J.-P., Plichart, R., Roux, J. and Grosset, J.-H. The implementation of chemoprophylaxis of leprosy in the Southern Marquesas with a single dose of 25 mg per kg rifamin. Int. J. Lepr. 57(1989)810-816.
9. Chanteau, S., Cartel, J.-L., Celerier, P., Plichart, R., Desforges, S. and Roux, J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int. J. Lepr. 57(1989)735-743.
10. Chanteau, S., Cartel, J.-L., Roux, J., Plichart, R. and Bach, M.-A. Comparison of synthetic antigens for detection of antibodies to phenolic glycolipid I in patients with leprosy and their household contacts. J. Infect. Dis. 4(1988)770-776.
11. Chanteau, S., Plichart, R., Boutin, J.-P., Roux, J. and Cartel, J.-L. Finger-prick blood collection and computer-assisted enzyme-linked immunosorbent assay for large-scale serological studies on leprosy. Trans. R. Soc. Trop. Med. Hyg. 83(1989)414-416.
12. Cho, S.-N., Fujiwara, T., Hunter, S. W., Rea, T. H., Gelber, R. H. and Brennan, P. J. Use of an artificial antigen containing the 3, 6-di-o-meth-yl-/3-d-gIucopyranosyl epitope for the serodi-agnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
13. Cho, S.-N., Hunter, S. W., Gelber, R. H., Rea, T. H. and Brennan, P. J. Quantitation of the phenolic glycolipid of M. leprae and relevance to glycolipid antigencmia in leprosy. J. Infect. Dis. 153(1986)560-569.
14. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. Seroepidemi-ological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
15. Gaylord, H. and Brennan, P. J. Leprosy and the leprosy bacillus: recent developments on characterization of antigens and immunology of the disease. Ann. Rev. Microbiol. 41(1987)645-675.
16. Noordeen, S. K. The epidemiology of leprosy. In: Leprosy. Hastings, R. C.,ed. London: Churchill Livingstone, 1985, pp. 15-30.
17. WHO Study Group. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
1. M.D.; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
2. Ph.D.; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
3. M.D.; Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
4. Computer Scientist, Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
5. M.D., Professor, Institut Louis Malarde, B. P. 30, Papeete, Tahiti, French Polynesia.
6. M.D., Head, Public Health Service in Southern Marquesas, Atuona, Hiva Oa, French Polynesia.
7. M.D., Professor, Faculté de Médecine Pitie-Salpe-triere, 91 Blvd. de l'Hôpital, 75613 Paris, France.
Received for publication on 19 October 1989.
Accepted for publication in revised form on 16 March 1990.