- Volume 58 , Number 3
- Page: 518–25
A study of autoantibodies in chronic mycobacterial infections
ABSTRACT
Infections can cause autoantibody production. The purpose of this study was to determine the prevalence of autoantibodies in patients with chronic mycobacterial infections. Sera f rom 41 leprosy patients and f rom 49 untreated and 73 treated tuberculosis (TB) patients were tested for the presence of rheumatoid factor, antinuclear factor, and several other autoantibodies. The rheumatoid factor, measured by the RheumaTec RF latex test, was positive in 2.4% of the leprosy patients and 2.7% of the treated TB patients but absent in the untreated TB group. The titers ranged f rom 40 to 160 international units. Positivity was dependent upon the technique utilized, and existed in 21% of untreated TB group and 4% of the treated TB patients when using the Rheuma-Wellcotest technique. The antinuclear antibody was positive in 7.3% of the leprosy group, 6.1% of the untreated TB group, and 15% of the treated TB patients (p = 0.0125). Antinuclear antibody positivity correlated with the duration of treatment of the TB patients (p = 0.025). The antinuclear antibody titers were low and gave no specific pattern on staining. No patient had antibodies against native deoxyribonucleic acid, ribonuclear protein, Ro (SS-A) or La (SS-B) antigens.Due to their low prevalence and frequency in these chronic infections, these autoantibodies should not lead to confusion in distinguishing these conditions f rom the connective tissue diseases.
RÉSUMÉ
Des infections peuvent engendrer une production d'auto-anticorps. Le but de cette étude était de déterminer la prévalence d'auto-anticorps chez, des patients avec des infections mycobactériennes chroniques. Les sérums de 41 patients lépreux, de 49 tuberculeux non traités, et de 73 tuberculeux (TB) traités ont été testés pour la fréquence de facteur rhumatoïde, facteur antinucléaire, et différents autres auto-anticorps. Le facteur rhumatoïde mesuré par le test au latex Theumatec RF, était positif chez 2,4% des malades de la lépre, et 2,7% des tuberculeux traités, mais absent dans le groupe des tuberculeux non traités. Les titres variaient de 40 à 160 unités internationales. La positivité dépendait de la technique utilisée, et était présente chez 21% des tuberculeux traités quand la technique du Thcuma-Wellcotest était utilisée. Le facteur antinucléaire était positif chez 7,3% des lépreux, 6,1% des patients TB non traités; et 15% des tuberculeux traités (p = 0,0125). La positivité du facteur antinucléaire était liée à la durée du traitement des patients TB (p = 0,025). Les titres du facteur antinucléaire étaient bas et ne donnaient aucune image spécifique à la coloration. Aucun patient ne présentait d'anticorps vis-à-vis de l'acide désoxyribonucléique naturel, la ribonucléoprotide, les antigènes Ro (SS-A) ou La (SS-B). A cause de leurs prevalence et frequence basses dans ces infections chroniques, ces auto-anticorps ne devraient pas mener a confondre ces conditions des maladies du tissu conjonctif.RESUMEN
Las infecciones pueden causar la producción de au-toanticuerpos. El propósito de este estudio fue el determinar la prevalência de autoanticuerpos en pacientes con infecciones micobacterianas crónicas. Los sueros de 41 pacientes con lepra, de 49 pacientes con tuberculosis (TB) no tratada y de 73 pacientes con tuberculosis tratada, se probaron para buscar la presencia de factor reumatóide, factor antinuclear, y otros autoanticuerpos. El factor reumatóide, medido con el reactivo RheumaTec RF fue positivo en el 2.4% de los pacientes con lepra y en el 2.7% de los pacientes con tuberculosis tratada pero fue negativo en los pacientes con TB no tratada. Los títulos variaron de 40 a 160 unidades internacionales. Con la ténica del Rheuma-Wellcotest, la positividad fue del 21% en el grupo TB no tratado y del 4% en los pacientes TB tratados. Los anticuerpos antinucleares fueron positivos en el 7.3% de los pacientes con lepra, en el 6.1% del grupo TB no tratado y en el 15% de los pacientes TB tratados (p = 0.0125). La positividad de los anticuerpos antinucleares correlacionó con la duración del tratamiento de los pacientes con TB (p = 0.025). Los títulos de anticuerpos antinueleares fueron bajos y no dieron un patrón específico de tinción. Ningún paciente tuvo anticuerpos contra DNA nativo, contra proteína ribonuclear ni contra los antígenos Ro (SS-A) o La (SS-B).Debido a su baja prevalência y frecuencia en estas infecciones crónicas, estos autoanticuerpos no afectan la diferenciación entre estas condiciones y las enfermedades del tejido conectivo.
Infectious diseases are common in black South Africans, and tuberculosis (TB) is particularly prevalent. It has previously been reported that infections arc associated with the induction of autoantibodies which, with the systemic manifestation of these common conditions, may lead to difficulties in differentiating them from the connective tissue diseases. To complicate matters further, controversy existed in previous reports regarding the prevalence of autoantibodies in mycobacterial infections. In fact, the association between autoimmunity and infection is currently being reassessed; recent reports have suggested that mycobacterial infection may play a role in the pathogenesis of autoimmune disorders (10,15,21,27). This study was undertaken solely to discover the prevalence of certain autoantibodies in the chronic mycobacterial infections, TB and leprosy.
The prevalence of circulating rheumatoid factor (RF), antinuclear (ANA), anti-dou-ble-stranded DNA (dsDNA), and anti-ex-tractable nuclear antigen (ENA) antibodies in the sera of black South African patients with pulmonary TB and leprosy infections was investigated.
PATIENTS AND METHODS
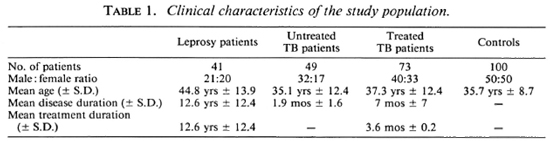
Patients. Four groups of patients were included in the study. Table 1 summarizes the demographic data. Group 1 comprised 41 consecutive black inpatients with leprosy from Westford Hospital, Pretoria. Applying the Ridley and Jopling classification (22), they comprised 18 patients with polar lepro-matous, 10 with borderline lepromatous, 7 with borderline tuberculoid, 4 with borderline, and 2 with polar tuberculoid leprosy. Complications occurred in 6 patients; 5 had erythema nodosum leprosum, 1 had a reversal reaction. Most patients required treatment with clofazimine, dapsone or rifampin. No patient had any associated medical disease.
Forty-nine consecutive black pulmonary TB patients (Group 2) were studied. They had presented to the Hillbrow and Baragwa-nath Hospitals with a clinical history, physical examination, and radiological features compatible with pulmonary TB. All patients had Mycobacterium tuberculosis documented on sputum examination.
Group 3 comprised 73 black pulmonary TB inpatients receiving treatment at the Rietfontein Hospital, Johannesburg. In addition to having the clinical and radiological features of pulmonary TB, all of these patients had had bacteriological confirmation of M. tuberculosis in the sputum at the time of diagnosis. Sixty-five patients were being treated with isoniazid (INH) and other drugs; eight patients were not treated with INH.
In Group 4 (the control group), 100 healthy black blood donors from the High-veld Blood Transfusion Service, Johannesburg, were selected to provide an age distribution similar to the TB Groups 2 and 3.
A clinical history was obtained and each patient was examined for associated disease. Patients with clinical evidence of connective tissue diseases were to be excluded from the study; however, no patient demonstrated such features.
The clinical details of the patients are demonstrated in Table 1. The patient groups were reasonably matched for age and sex, but the leprosy patients had mostly had protracted therapy. Informed consent was obtained from all of the patients participating in the study.
Rheumatoid factor (RF) was measured using both the RheumaTec® RF latex test (Labor Diagnostica, Federal Republic of Germany), which utilizes IgG-coated polystyrene beads, and the Rheuma-Wellcotest (Wellcome Reagents Ltd, United Kingdom).
Antinuclear antibody (ANA) measurements. ANA were measured by indirect immunofluorescence using as substrate 4-μm-thick sections of rat kidney and liver as well as a HEP-2 cell line (Kalestad Laboratories, Federal Republic of Germany). Antibodies against dsDNA were measured by indirect immunofluorescence using Crithidia luciliae as substrate. Extractable nuclear antigen contains RNA and a protein with two different reactive components. Antibodies against these two reactive components, ribonuclear protein (RNP) and Smith (SM; named after a patient), were investigated by counterimmune electrophoresis using rabbit thymus powder as substrate; other autoantibodies, particularly prevalent in Sjögrens syndrome, anti-SSA (Ro) and anti-SSB (La), were assessed using an Ouchterlony double-immune gel-diffusion technique (Poly ENA commercial kit; Zeus Laboratories, Federal Republic of Germany), according to the manufacturer's instructions.
RESULTS
Rheumatoid factor. The prevalence and titer of the RF measured, using the RhcumaTec® RF latex test, were low in the population studied. In the leprosy group (41 patients) only one patient had a positive RF of 40 International Units (IU), and in the treated TB group (73 patients) two patients had RF with titers of 80 IU and 160 IU, respectively. In the untreated TB group (49 patients) and the control group (100 patients), all patients were RF negative.
In addition, 43 scrum samples (19 untreated TB patients and 24 treated TB patients) were randomly selected for comparative testing with the Rheuma-Wellcotest RF test. Using this technique, four of the untreated TB patients (21%) had a positive RF ranging from 1:20 to 1:40 compared with only one treated TB patient (4%) with a titer of 1:40. Because of the small sample population, this difference was not statistically significant (Yates x2 1-53; p = 0.23). However in the untreated TB patients, a comparison of the two detection systems (Rheuma-Wcllcotest and RheumaTec® RF) for RF positivity suggested borderline statistical significance (Yates x2 2.51; p = 0.05).
Antinuclear antibodies. Table 2 shows that the prevalence of ANA in the leprosy group was 7.3% and although higher than in the control group (2%), it was not statistically significant. ANA positivity did not correlate with reactional states. In the TB patients, however, the treated patients did have a significantly increased ANA positivity (15.1 %). It was noted that the eight treated patients who did not receive INH were all ANA negative. Conversely, 17% of the 65 patients treated with INH were ANA positive. Table 2 also shows the range of titers found. Although these were mostly low, they were as high as 1:640 in one of the treated patients. Although the speckled pattern was the one most frequently seen, there was no uniformity within the groups.
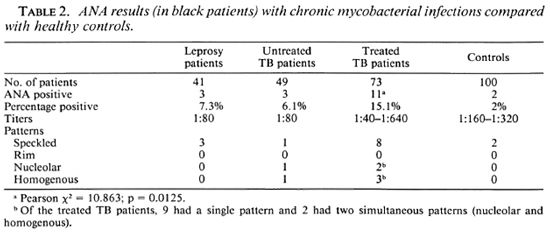
In TB patients ANA positivity was associated with treatment duration (mean duration of treatment of positives vs mean duration of treatment of ANA negatives p = 0.036), as well as being more significant for those patients receiving INH therapy (p = 0.026).
Of interest is the fact that none of the serum samples reacted against native DNA, SS-a, SS-b, RNP, or Sm antigens; nor did any of the patients with or without ANA show features of a connective tissue disease.
DISCUSSION
Bonomo, et al. (5) were the first workers to describe autoantibodies in leprosy. Subsequently, the frequent occurrence of autoantibodies in patients with leprosy has been documented in a number of studies, and this work adds to the extensive literature on the subject. However, this is the first study dealing with the prevalence, pattern, and titers of autoantibodies in black South African patients. Moreover, in 1967 Bonomo, et al. (4) described a 42-year-old female with lupoid features who had positive RF, ANA and LE cell phenomenon, suggesting that a clinical overlap exists between leprosy and diseases with autoimmune phenomena, in particular systemic lupus erythematosus (SLE).
Discrepancy exists in the literature about the prevalence of RF in leprosy patients. Some investigators found up to 50% of leprosy patients RF positive (1), while others have failed to detect such autoantibodies (14).
A number of factors must be considered, including the type of assay utilized, genetic factors, study population, the type and severity of the disease, and the possible role of different treatment regimens. Unfortunately, since leprosy is an uncommon disease and the mean disease duration in this study was 12 years, we may have missed the immunological aberrations related to the patients' early disease and treatment.
Difficulty exists in assaying RF because the test has not been standardized. IgM RFs are multivalent antibodies and effective ag-glutinators of antigen-coated particles, such as bentonite or latex beads, which have been passively coated with human IgG. The cross-linking of these IgG-coated particles by IgM RF in serum produce a fiocculation reaction. In addition technical problems have arisen, particularly in chronic infections where patients arc hypergammaglobuli-nemic, because the IgG antigen with which RF reacts may crossreact or interfere with a specific detection system. Positivity may vary according to the size and characteristics of the particles used.
The Rheuma-Tec® RF latex test utilized in this study is an aqueous suspension of polystyrene particles coated with human gamma globulin. The positivity of the patients with mycobacterial infections who were tested was shown to be low. This is in contrast to the results obtained when using the Rheuma-Wellcotcst system, where 21% of the untreated TB patients tested demonstrated a positive RF. The tests differ: in the Rheuma-Tec® RF test the patient serum is diluted in a physiological solution (saline 0.9%); the Rheuma-Wellcotest utilizes a glycine buffer as diluent.
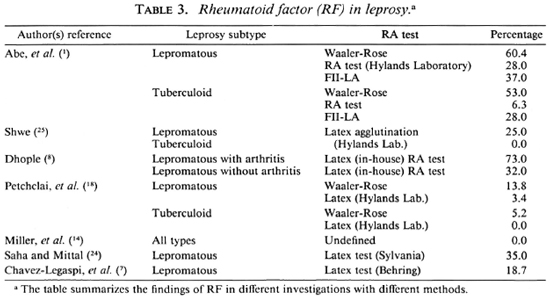
When comparing the results of the two systems, the Rheuma-Wellcotest produced a higher positivity. Furthermore, the positivity of RF is dependent on the technique used, as documented by other investigators and summarized in Table 3 (1,8,18). From the results obtained in this study, it appears that the Rheuma-Tec® RF system produces a low frequency of crossreactivity (false-positives) in chronic infections.
In addition, Reyes, el al. (20) demonstrated that RF positivity in TB patients was dependent upon disease severity. It may be possible that patients studied by other workers had more severe disease than those patients in the present study population.
Table 3 shows that the low prevalence of RF found in this study is similar to that of the tuberculoid patients in the study of Shwe (25), and of the whole group in the studies of Petchclai, et al. (18) and Miller, et al. (14). Petchclai, ct al. (18) suggested that the low occurrence in their study could be due to an alteration in the immune response among the Thai subjects. Contradictory results have been obtained by Abe, et al. (1), Saha and Mittal (24), and Dhople (8) who studied the prevalence of RF during and after an acute lepra reaction in 39 patients. In Dhople's study, the patients were divided into two groups-those with acute lepra reaction with arthritis (11) and those with acute lepra reaction without arthritis (28) (8). He found that 73% of the patients in the first group (8 of 11 patients) had a positive RF during the acute attack compared with 32% (9 of 28) with positive RF in the second group (8). His conclusion was that RF may play a role in the pathogenesis of arthritis and the rheumatological manifestations that patients with acute lepra reactions frequently experience. However, in contrast to Dhople's study, in the present study five patients had ENL, and none had a positive RF. In addition, our group contained a significant proportion of the lepromatous-type patients who did not demonstrate a high frequency of RF positivity.
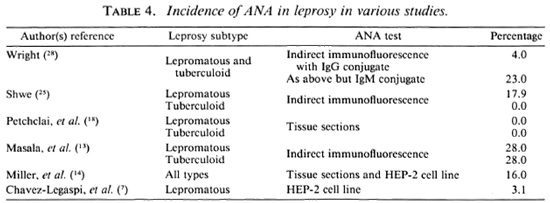
From Table 4 it can be seen that there was also quite a wide variation in the reported occurrence of ANA in leprosy patients, ranging from absent or very low, as in the present study, up to the 28% reported by Masala, et al. (13). Shwe found ANA more common in the lepromatous patients (25), while in Masala's groups it was equally as common in the tuberculoid group (13). The low sensitivity of the ANA tests used in the present study is unlikely to be the reason for the low incidence. The tests have been used routinely in our lupus clinics, and over a number of years been found to be reliable.
In two separate studies of the presence of RF in TB patients (6,12), a higher prevalence of RF positivity was demonstrated. Twenty-four percent of the untreated and 28.8% of the treated TB patients had a positive RF (6). Lindqvist, et al. (12) demonstrated in their study that RF was positive in 40% of patients, with titers ranging from 1:20 to 1:640. None of the untreated patients in the present study had RF, and it was present in only 2.8% of the treated TB group. As mentioned previously, the laboratory technique and possible genetic factors may account for this discrepancy.
ANA may be produced in the body as a response to a number of drugs; however, only a small proportion of patients who produce ANA as a result of drugs develop clinical SLE (2). In the present study, examination of all patients failed to demonstrate any clinical features of SLE. This is borne out by evidence from the Connective Tissue Disease Clinic at Hillbrow Hospital, Johannesburg, where, in our experience, only one equivocal case of drug-induced lupus was found out of a group of approximately 100 black SLE patients. Furthermore, one must remember that in the United States of America drug-induced lupus is a rare condition among blacks (9).
Among the untreated TB population only 6.1% had a positive ANA test compared with 17% of the treated TB group. However, none of the scrum samples reacted against dsDNA, anti-Sm, anti-SSA, anti-SSB, or anti-RNP.
We found that the appearance of ANA correlated with treatment duration and not with disease duration, suggesting that ANA was induced mainly by antituberculous drugs, in particular INH. None of the patients treated with drugs other than INH had a positive ANA test. Titers were generally low, except for one patient whose ANA titer was 1:640. ANA was more prevalent in males than in females, this difference not being statistically significant but noteworthy, considering the predominence of females suffering from SLE and drug-induced SLE.
The induction of ANA by antituberculous drugs was first described by Cannat and Seligmann (6). In their study the incidence of ANA in 125 untreated TB patients was compared to that of 75 treated TB patients. The prevalence of ANA was 4% in the untreated group and 20% in the treated group (6). These findings are similar to those of the present study. However, the authors did not comment on titers and did not analyze sex, age, and treatment duration in relation to the induction of ANA (6).
In a prospective study, Rothfield, et al. (23) also demonstrated the effects of treatment on ANA induction. Of 192 pulmonary TB cases, 4% were ANA positive before treatment and 21.6% developed ANA during treatment (23). The role of the duration of treatment was not analyzed.
Thewaini Ali (26) found only 4 out of 80 TB patients to have low titers of ANA. Lindqvist, et al. (12) found that 46% of 60 TB patients had a positive ANA. However, all of their patients were receiving TB treatment, and the study did not divide the patients into treated or untreated TB (12).
It was demonstrated by Price-Evans that INH and hydralazine were polymorphically metabolized in humans (19). Perry, et al. (16,17) and Alarcón-Segovia, et al. (2) have demonstrated that the presence of ANA is more prevalent in patients who phenotyp-ically are slow acetylators. Alarcón-Segovia, et al. (2) analyzed the incidence of ANA in 184 treated TB patients. However, in contrast to our findings, their study showed a higher prevalence of ANA in females (2). In a different study, the same workers analyzed 153 TB patients receiving INH therapy (3). Of this group of patients, 78 were slow and 75 rapid acetylator phenotypes; the overall ANA positivity was 20% (3). In our current study, the patterns of acetylation in black South Africans were not analyzed, but it has been demonstrated previously that the prevalence of both slow and fast acetylator phenotypes is similar in black and white South Africans, with the exception of the Kgalagadi of the Sotho/Tswana tribe who have a low frequency of slow acetylation phenotype (11). There were no Kgalagadi in the present study population.
Acknowledgments. This study was made possible by a financial grant from the Department of Immunology, South African Institute for Medical Research, and a grant from the Rheumatology Division, Department of Medicine, University of the Witwaters-rand, Johannesburg, Republic of South Africa.
The authors wish to thank Dr. A. J. Gear for his guidance and support while collecting the research data for this project, Oria Cohen for her secretarial assistance, and Sr. Lee-Anne Rapoport for her help in drawing blood.
REFERENCES
1. Abe, M., Chinone, S. and Hirako, T. Rheumatoid-factor-like substance and antistreptolysin O antibody in leprosy serum; significance in erythema nodosum leprosum. Int. J. Lepr. 35(1967)336-344.
2. Alarcon-Segovia, D., Fishbein, E. and Alcala, H. Isoniazid acetylation rate and development of antinuclcar antibodies upon isoniazid treatment. Arthritis Rheum. 14(1971)748-752.
3. Alarcon-Segovia, D., Fishbein, E. and Be-tancourt, V. M. Antibodies to nuclcoprotcin and to hydrazide-altered soluble nucleoprotein in tuberculous patients receiving isoniazid. Clin. Exp. Immunol. 5(1969)429-437.
4. Bonomo, L., Dammacco, F., Tursi, A. and Barbieri, G. Lupoid features in a case of leprosy. Int. J. Lepr. 35(1967)65-71.
5. Bonomo, L., Tursi, A., Trimigliozzi, G. and Dammacco, F. L.E. cells and antinuclear factors in leprosy. Br. Med. J. 2(1965)689-690.
6. Cannat, A. and Seligmann, M. Possible induction of antinuclear antibodies by isoniazid. Lancet 1(1966)185-187.
7. Chavez-Legaspi, M., Gomez-Vazquez, A. and Garcia-De La Torre, I. Study of rheumatic manifestations and serologic abnormalities in patients with lepromatous leprosy. J. Rheumatol. 12(1985)738-741.
8. Dhople, A. M. Possible autoimmune phenomenon in leprosy. Jpn. J. Exp. Med. 42(1972)125-129.
9. Hess, E. Drug-related lupus. N. Engl. J. Med. 318(1988)1460-1462.
10. Holoshitz, J., Klajman, A., Drucker, I., Lapi-dot, Z., Yaretzky, A., Frenkel, A., van Eden, W. and Cohen, I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet 2(1986)305-309.
11. Jenkins, T. Genetic variation and disease in southern African peoples. In: Variation, Culture
13. Masala, C, Amendolea, M. A., Nuti, M., Ric-and Evolution in African Populations. Singer, R. and Lundy, J. K., eds. Johannesburg: Witwaters-rand University Press, 1986, pp. 143-158.
12. Lindqvist, K. J., Coleman, R. E. and Osterland, K. C. Autoantibodies in chronic pulmonary tuberculosis. J. Chron. Dis. 22 (1970) 717-725. carducci, R., Tarahini, C. G. L. and Tarabini, C. G. Autoantibodies in leprosy. Int. J. Lepr. 47(1979)171-175.
14. Miller, R. A., Wener, M. H., Harnisch, J. P. and Gilliland, B. C. The limited spectrum of antinuclcar antibodies in leprosy. J. Rheumatol. 14(1987)108-110.
15. Ottenhoff, T. H. M., Torres, P., Terencio de las Aguas, J., Fernandez, R., van Eden, W., de Vries, R. R. P. and Stanford, J. L. Evidence for an HLA-DR4-associated immune-response gene for Mycobacterium tuberculosis. Lancet 2(1986)310-312.
16. Perry, H. M., Jr., Sakamoto, A. and Tan, E. M. Relationship of acetylating enzyme to hydralazine toxicity. (Abstract) J. Lab. Clin. Med. 70(1967)1020-1021.
17. Perry, H. M., Jr., Tan, E. M., Carmody, S. and Sakamoto, A. Relationship of acetyl transferase activity to antinuclear antibodies and toxic symptoms in hypertensive patients treated with hydralazine. J. Lab. Clin. Med. 76(1970)114-125.
18. Petchclai, B., Chuthanondh, R., Rungruong, S. and Ramasoota, T. Autoantibodies in leprosy among Thai patients. Lancet 1(1973)1481-1482.
19. Price Evans, D. A., Bullen, M. F., Houston, J., Hopkins, C. A. and Vetters, J. M. Antinuclear factor in rapid and slow acetylator patients treated with isoniazid. J. Med. Genet. 8(1972)53-56.
20. Reyes, P. A., Maluf, J. G., Curd, J. G. and Vaughan, J. H. Association of rheumatoid factor with complement activation in rheumatoid arthritis and other diseases. Clin. Exp. Immunol. 53(1983)391-396.
21. Rheumatoid arthritis and tuberculosis. (Editorial) Lancet 2(1986)321-322.
22. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
23. Rothfield, N. F., Bierer, W. F. and Garfield, J. W. Isoniazid induction of antinuclcar antibodies; a prospective study. Ann. Intern. Med. 88(1978)650-652.
24. Saha, K. and Mittal, M. M. Immunologic aspects of lepromatous leprosy with special reference to the study of autoantibodies. Int. J. Lepr. 40(1972)260-264.
25. Shwe, T. Clinical significance of autoimmune antibodies in leprosy. Trans. R. Soc. Trop. Med. Hyg. 66(1972)749-753.
26. Thewaini Ali, A. J. Auto-antibodies in patients with chronic pulmonary tuberculosis. J. Med. Microbiol. 2(1969)566-570.
27. van Eden, W., Holoshitz, J., Nevo, Z., Frenkel, A., Klajman, A. and Cohen, I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc. Natl. Acad. Sci. U.S.A. 82(1985)5117-5120.
28. Wright, D. J. M. Autoantibodies in leprosy. (Letter) Lancet 2(1973)40.
1. M.D., M.Med., Division of Rheumatology, Department of Medicine, University of the Witwatersrand, Johannesburg, South Africa.
2. M.B.B.Ch., M.Med., Division of Rheumatology, Department of Medicine, University of the Witwatersrand, Johannesburg, South Africa.
3. M.B.B.Ch., Ph.D., Serology Department, South African Institute for Medical Research, Johannesburg. South Africa.
4. Serology Department, South African Institute for Medical Research, Johannesburg. South Africa.
Reprint requests to: Dr. B. L. Rapoport, Department of Medical Oncology, H. F. Verwoerd Hospital, Private Bag X 169, Pretoria 0001, Republic of South Africa.
This study forms part of a dissertation submitted to the University of Witwatersrand, Johannesburg, South Africa, for the degree of Master of Medicine.
Received for publication on 16 October 1989.
Accepted for publication in revised form on 14 February 1990.