- Volume 58 , Number 3
- Page: 526–33
Uptake of purine and pyrimidine nucleosides by macrophage-resident Mycobacterium leprae: 3H-Adenosine as an indicator of viability and antimicrobial activity
ABSTRACT
Freshly extracted human- and armadillo-derived Mycobacterium leprae maintained within murine macrophages incorporated significant levels (p < 0.05 to p < 0.001) of 3H-adenosine and 3H-hypoxanthine by 6 and 9 days of the culture period. The incorporation of 3H-adenosine was twofold or more higher than 3H-thymidine in 10 out of 15 human-derived M. leprae isolates. Macrophage-adapted bacilli incorporated 10-14-fold higher levels of 3H-adenosine compared to the same bacilli maintained in axenic cultures. The incorporation of these two labels was inhibited by dapsone and rifampin, indicating the utility of in vitro radiometric assays for screening antileprosy drugs and drug sensitivity/resistance in patients.RÉSUMÉ
Des Mycobacterium leprae fraîchement récoltés chez l'homme et le tatou et maintenus dans des macrophages de souris, incorporaient des taux significatifs (p < 0,05 à p < 0,001) de 3H-adénosine et 3H-hypo-xanthine aux sixième et neuvième jours de la période de culture. L'incorporation de 3H-adénosine était deux fois plus élevée ou davantage que celle de 3H-thymi-dine dans 10 des 15 isolats de M. leprae d'origine humaine. Les bacilles adaptes aux macrophages incorporaient des taux de 3H-adénosinc 10 à 14 fois plus élevés que les mêmes bacilles maintenus dans des cultures axéniques. L'incorporation de ces deux marqueurs était empêchée par la dapsone et la rifampicine, indiquant l'utilité de titrages radiométriques in vitro pour l'étude de médicaments anti-lèpre et de la sensibilité/résistance aux médicaments chez les patients.RESUMEN
Las células de Mycobacterium leprae recién extraídas de lesiones humanas y de armadillos y mantenidas dentro de macrófagos murinos, incorporaron niveles significativos de adenosina-3H y de hipoxantina-3H (p < 0.05 a p < 0.001) durante 6 y 9 días del periodo de cultivo. La incorporación de adenosina-3H fue 2 o más veces mayor que la incorporación de timidina-3H en 10 de 15 muestras de M. leprae obtenidas de humanos. Los bacilos adaptados a los macrófagos incorporaron de 10 a 14 veces más adenosina-3H que los mismos bacilos mantenidos en cultivos axénicos. La incorporación de estas dos marcas se inhibió por dapsona y por rifampina. Esto indica la utilidad de los ensayos radimétricos in vitro para evaluar la efectividad de drogas antileprosas y la sensibilidad o resistencia de M. leprae a las mismas.The inability to cultivate Mycobacterium leprae and to conveniently evaluate its viability has been a major constraint for the development of antileprosy drugs. The commonly used mouse-foot-pad technique requires 6-12 months and is dependent on counting acid-fast bacilli extracted from tissues (2,21)- To overcome these disadvantages, several in vitro methodologies have been developed in the past decade. These include primarily the uptake of various radiolabeled precursors indicative of M. leprae metabolism (1,4-6,12-14,17,28) staining with fluorescenating dyes (8), cationic ratio (20), and adenosine triphosphate measurements (3). The incorporation of radiolabels has been studied in axenic cultures as well as in tissue culture systems (7,13,14,17). In addition, the effect of live M. leprae on the modulation of macrophage cell-surface molecules has been used as a monitor of viability (10).
Since the leprosy bacillus has been shown to reside naturally within mononuclear cells, our laboratory had developed a macro-phage-based radiometric assay whereby the uptake of 3H-thymidine was used as an indicator of DNA synthesis of M. leprae resident within these cells (18). Both human (15) and murine macrophage cultures (11,18) were experimentally infected with human- and armadillo-derived M. leprae, and the radiolabel was shown to be selectively incorporated in the bacillus by autoradiography (11). In addition, this methodology was amenable to screening antilcprosy drugs (11), drug resistance (19), and the effect of lymphokines (16), and compared well with the mouse-foot-pad model (19). The assay is completed in 2 weeks and requires fewer bacilli (2 x 105) than axenic cultures which require 106 to 1010 bacilli. This has a distinct advantage in studying patients where ethical constraints on biopsy size limit the number of bacilli obtained from the skin. Moreover, the human infection rarely attains the bacillary levels seen in the armadillo. Further reduction of bacilli is seen in patients under chemotherapy. In addition, macrophages as effector cells would be a good model for dissecting the host-resistance factors relating to antimicrobicidal activity (16).
The present study was undertaken to explore alternative radiolabeled nucleosides that would be selectively incorporated into M. leprae. Both purines and pyrimidines were used. Of these, 3H-adenosine and 3H-hypoxanthine showed higher incorporation as compared to 3H-thymidine. The former was incorporated maximally and was useful in in vitro screening of antileprosy drugs. Preliminary results suggest that macrophage-adapted bacilli are better incorporators of 3H-adenosine than bacilli maintained in axenic cultures.
MATERIALS AND METHODS
Patients. Twenty-seven, bacilliferous, lepromatous leprosy patients undergoing multidrug therapy in the endemic district of Baroda, Gujarat, and the Hansen's clinic of Safdarjang Hospital, New Delhi, India, were selected for this study. Routine excision or 6-mm punch biopsies (Laboratories Stefel, UK Ltd., Slough, England) were obtained under sterile conditions, air freighted over ice to the laboratory within 12-14 hr, and stored at - 70ºC until further processing.
Extraction of M. leprae. M. leprae were extracted under sterile conditions by tissue homogenization. In brief, biopsies were washed twice in RPMI 1640 medium (GIB-CO Biocult, Irvine, Scotland), and the dermal tissue separated after removal of subcutaneous fat. The tissue was minced and subsequently homogenized using a Mickle disintegrator (The Mickle Laboratory Engineering Co. Ltd., Gomshall, Surrey, England) with a vibrational frequency of 50 shakes per sec for 5 min, each shake having a 3-mm excursion. The homogenate was centrifuged at a low speed of 40 x g x 10 min (Sorvall RT-8000) to remove coarse tissue fragments. The supernatant consisting of the bacillary suspension was counted by the method of Shepard and McRae (22).
All M. leprae extracts were checked for the absence of cultivable contaminants by plating on nutrient agar and Lowenstein-Jensen (LJ) medium. Isolates showing no growth at 24 hr on the former were inoculated in macrophage cultures. However, the nutrient agar and LJ slants were maintained up to 48 hr and 8 weeks, respectively. No cultivable organisms were detected in the extracts used in the present study.
Macrophage cultures. Cultures of murine peritoneal macrophages were set up in 96-well microtiter plates as described earlier (11). In brief, resident peritoneal macrophages from BALB/c mice were obtained by peritoneal lavage and mixed with an equal volume of RPMI 1640 medium supplemented with 20% fetal calf serum (FCS; GIBCO) and incubated at 37ºC for 12-18 hr. The nonadherent cells were removed and the wells were replaced with RPMI 1640 supplemented with 20% FCS. Wells were infected at this stage with 200 μl/ml of freshly extracted M. leprae. Cultures were maintained for 16-18 hr at 37ºC in 5% CO2 in air. Nonphagocytosed bacilli were replaced with media containing various radiolabels (1 μCi/ml). For the drug-inhibition studies, varying concentrations of drugs (3-100 ng/ ml) were added along with the radiolabel. The cultures were harvested onto glass fiber discs (GF/c; Whatman Inc., Clifton, New Jersey, U.S.A.) using a cell harvester (Ph.D. Cell Harvester, Cambridge Technology, Inc., Watertown, Massachusetts, U.S.A.) at the time period specified. The cells were incubated in 1 N NaOH and serially flushed with distilled water, normal saline containing cold nucleosides (1 mg/ml), 50% cold trichloroacetic acid (TCA), and fresh ethanol. The discs were dried, placed in scintillation mixtures, and counted in a beta scintillation counter (1217 Rack Beta, LKB, Finland). Using the same batch of macrophages, five replicates of the following were set up: Macrophage + a) freshly extracted M. leprae, b) heat-killed M. leprae, c) a + varying concentrations of dapsone and rifampin. The percent inhibition in the presence of drugs was calculated as follows:
% inhibition = 1 - (mean cpm of live M. leprae cultures + drug/mean cpm of live M. leprae cultures) x 100.
The p value was calculated by the Mann-Whitney U test.
Radiolabels and drugs. The incorporation of three radiolabeled purines-2, 5, 8, 3H-adenosine (sp. act. 49 Ci/mmol); de oxy-1, 2, 5, 8,3H-adenosine (sp. act. 68 Ci/ mmol); and 3H-hypoxanthine (sp. act. 26 Ci/mmol) - and three radiolabeled pyrimi-dincs-5-3H-cytidinc (sp. act. 26 Ci/mmol); 3H-dcoxy-cytidine (62 Ci/mmol); and 5, 6, 3H-uracil (sp. act. 46 Ci/mmol) -and methyl3H-thymidinc (Amersham International, Buckinghamshire, England) were studied in M. leprae resident in macrophage cultures. These compounds were diluted in RPMI 1640 medium and used at 0.25 μCi/ well.
Rifampin and dapsone were dissolved in ethylene glycol and 1 N HC1, respectively, at a stock concentration of 1 mg/ml. The drugs were diluted in RPMI 1640 to 1 μg/ ml, and sterilized by passage through 0.22-μm Millipore discs prior to use.
Axenic cultures. The cell-free incorporation studies using specialized in vitro media were done as described by Khanolkar and Wheeler (7) with a minor modification in the temperature used. In brief, 1010 M. leprae were incubated aseptically in specialized medium (7) for 24 hr at 37ºC. After incubation, the bacteria were suspended in 10 ml of 0.1% Tween 80 (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) and cen-trifuged at 5000 x g x 1 hr. The pellet was washed three times with 5% TCA and twice with methanol, and the radioactivity was estimated as before in a beta scintillation counter.
Comparison of axenic and macrophage cultures. The same batch of M. leprae extracts were tested in parallel in both types of cultures. Due to the lower yield of bacilli, biopsies from six patients were pooled together; whereas the same armadillo tissue was used for isolating M. leprae. The axenic cultures were maintained for the first 24 hr only; macrophage cultures were continued for 9 days. The former had 1010 bacilli in 500 μl and the latter had 2 x 105 bacilli per 200 μl per well. As described above, the axenic cultures were in specialized medium (7) of pH 7; whereas macrophage cultures were set up in FCS-enriched RPMI 1640. Both types of cultures were maintained at 37ºC and harvested as above. Picomols of 3H-adcnosine/1010 bacilli/24 hr in axenic cultures was calculated as:

Δcpm = mean cpm of "live" M. leprae culture - mean cpm of heat-killed M. leprae.
The same calculations were done for the macrophage cultures except for computing the cpm obtained by correcting for the number of bacilli and expressing the results for 24 hr instead of cumulative uptake over 9 days. Correction was not made for the unknown numbers of nonphagocytosed bacilli.
RESULTS
Incorporation and time kinetics for radiolabeled nucleosides in M. leprae-resident macrophages. Shown in Figure 1 are the results obtained with (Fig. 1 A) four human-and (Fig. 1B) two armadillo-derived M. leprae isolates maintained in murine macrophage cultures in the presence of radiolabeled purines and pyrimidines. A time-related significant uptake of the purines 3H-adcnosinc and 3H-hypoxanthine (p < 0.001) was observed by 6-9 days of the culture period in macrophages with freshly extracted as compared to heat-killed M. leprae of the same isolates. Of these, 3H-adenosine showed a significantly higher incorporation (p < 0.01) at an earlier time of 6 days as compared to the 3H-hypoxanthine uptake which peaked at 9 days. In contrast, 3H-deoxyadenosine, as well as the radiolabeled pyrimidines cytidine, deoxycytidine and uracil, failed to show an appreciable uptake over the 2-weck culture period.
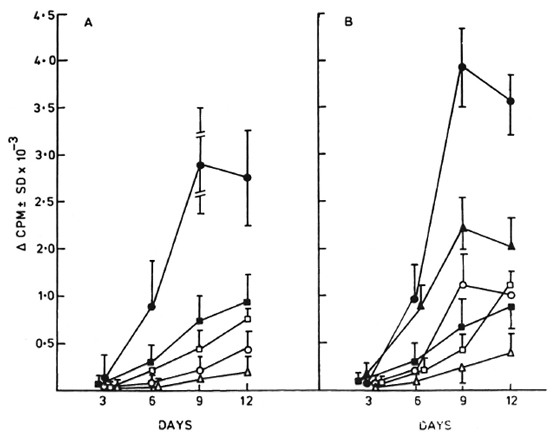
Fig. 1. Kinetics of uptake of radiolabeled purine and pyrimidine nucleosides by (A) four human- and (B) two armadillo-derived M. leprae isolates maintained in murine macrophage cultures. A time-related increase in net uptake was observed for 3H-adenosine ( ) and 3H-hyposanthine (▲) by days 9-12. 3H-deoxyadenosine (
) and 3H-hyposanthine (▲) by days 9-12. 3H-deoxyadenosine ( ), 3H-cytidine (
), 3H-cytidine ( ). 3H-deoxycytidine (
). 3H-deoxycytidine ( ), and 3H-uracil (Δ) failed to incorporate significantly over a similar culture period (Δcpm as in Materials and Methods).
), and 3H-uracil (Δ) failed to incorporate significantly over a similar culture period (Δcpm as in Materials and Methods).
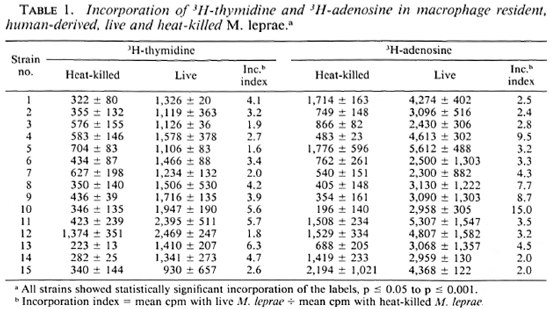
Comparison of uptake of radiolabeled adenosine and thymidine by same M. leprae isolates. M. leprae isolates derived from 15 bacilliferous leprosy patients (BL and LL) were tested in parallel cultures containing 3H-adenosine or 3H-thymidine (Table 1). Significant levels of incorporation of both radiolabcls were obtained in those cultures with live bacilli as compared to those with heat-killed bacilli from the same biopsy (p < 0.05 to < 0.001). In 10 of 15 isolates, the mean cpm of 3H-adenosinc was twofold or more higher than that of 3H-thymidine.
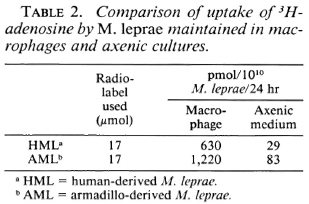
Comparison of uptake of 3H-adcnosine in M. leprae maintained in macrophage and axenic cultures. Comparative studies were undertaken on the same batch of pooled human- and a single armadillo-derived M. leprae isolate maintained in murine macrophages for 9 days and in axenic cultures for 24 hr. To make comparisons possible between the two assays, where different numbers of M. leprae and varying time periods were inherent to the methodology, the data obtained in the macrophage radiometric assay were computed to reflect the incorporation in axenic cultures where 1010 bacilli were used for a 24-hr period. The same 37ºC temperature was used for both assays in order to minimize, where possible, the variations in the two assays.
Table 2 shows the 10-14-fold greater uptake of 3H-thymidine in M. leprae maintained in macrophages as compared to the same isolates tested in the cell-free system. These data indicate that under the experimental conditions used, both human- and armadillo-derived bacilli adapted in host cells have a higher metabolic activity requiring adenosine than do free bacilli maintained in specialized medium.
Effect of antileprosy drugs. The inhibitory effects of dapsone and rifampin on the uptake of 3H-adcnosinc and 3H-hypoxan-thine were tested on six M. leprae isolates. In conformity with our earlier reports on 3H-thymidine uptake (11) it was observed that > 50% inhibition of uptake in drug-containing cultures gave a p value of < 0.05 to < 0.001 by the Mann-Whitney U test. Figure 2 shows the mean percent inhibition of 3H-adenosinc incorporation in three human- and two armadillo-derived M. leprae tested over 3-100 ng/ml of drugs. Dapsone showed significant inhibition (p < 0.05-< 0.001) at 10 ng/ml (Fig. 2A); whereas rifampin showed a similar effect at a lower concentration of 3 ng/ml (Fig. 2B). A graded increase in the level of inhibition was observable with the increase in drug concentration to 20-40 ng/ml, after which a plateau was reached.
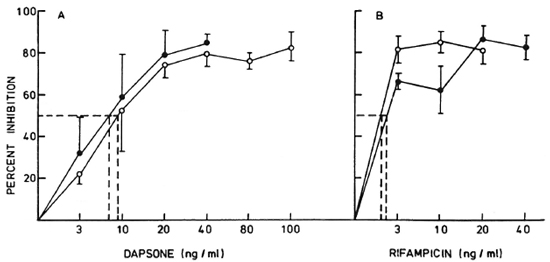
Fig. 2. Effect of varying concentrations of (A) dapsone and (B) rifampin on 3H-adenosine incorporation in three human-derived ( ) and two armadillo-derived (
) and two armadillo-derived ( ) M. leprae isolates residing in murine macrophage cultures. A linear increase in percent inhibition was observed from 3 ng/ml to 20 ng/ml of dapsone, followed by a plateau up to 100 ng/ml. Rifampin showed a significant inhibition (p < 0.01) at 3 ng/ml and failed to show a proportional increase up to 40 ng/ml; (---) = lowest concentration showing significant inhibition (p < 0.01-p < 0.05).
) M. leprae isolates residing in murine macrophage cultures. A linear increase in percent inhibition was observed from 3 ng/ml to 20 ng/ml of dapsone, followed by a plateau up to 100 ng/ml. Rifampin showed a significant inhibition (p < 0.01) at 3 ng/ml and failed to show a proportional increase up to 40 ng/ml; (---) = lowest concentration showing significant inhibition (p < 0.01-p < 0.05).
Figure 3 shows similar levels of inhibition of 3H-hypoxanthine and 3H-thymidine tested over a limited concentration range of 3 ng and 10 ng of dapsone in six human-derived M. leprae isolates. A wider range of drug concentrations was not tested due to constraints of numbers of bacilli obtainable from skin biopsies.
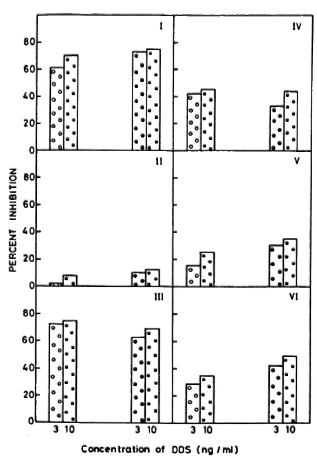
Fig. 3. Comparison of percentage of inhibition of 3H-hypoxanthinc with 3H-thymidine incorporation in six M. leprae isolates in the presence of dapsone. Roman numerals indicate individual bacillary isolates; each isolate was concurrently assayed for both radiolabels. Results arc shown for macrophage cultures pulsed with 3H-hypoxanthine with 3 ng/ml ( ) and 10 ng/ml (
) and 10 ng/ml ( ) of dapsone and for cultures pulsed with 3H-thy-midinc with 3 ng/ml (
) of dapsone and for cultures pulsed with 3H-thy-midinc with 3 ng/ml ( ) and 10 ng/ml (
) and 10 ng/ml ( ) of dapsone.
) of dapsone.
DISCUSSION
The present study confirms our earlier observations (19) regarding the suitability of the macrophage-based assay for monitoring the viability of M. leprae by the use of radiolabeled nucleosides. Of the purines and pyrimidines tested, 3H-adenosine showed a more rapid and higher level of incorporation by the leprosy bacillus. In general, in terms of the magnitude of mean cpm obtained, the order of uptake was ^-thymidine, 3H-hypoxanthine, and 3H-adenosine in increasing order. Comparative studies of the same isolates indicated that 10 of 15 human-derived bacilli showed a twofold higher incorporation of 3H-adenosine as compared to 3H-thymidine. A similar order was observed with regard to the time of incorporation of the three radiolabels. Whereas significant uptake of 3H-thymidine was usually observable by 14 days, 3H-hypo-xanthine and 3H-adenosine were incorporated at 9 and 6 days, respectively.
That the uptake was an indicator of the viability of the organisms was established by the significant increase in cpm in cultures with freshly extracted bacilli as compared to parallel cultures with heat-killed bacilli from the same isolates (p < 0.05 to 0.001). Moreover, 3H-adcnosine and 3H-hypoxan-thine uptake indicated metabolic activity of the bacilli since there was a graded increase in cpm over a period of 6 to 9 days of culture. This uptake was inhibited by antilep-rosy drugs.
Radiolabeled deoxyadenosine, cytidine, and deoxycitidine and uracil failed to be incorporated by M. leprae in our system. The first three compounds had also been shown to be ineffective in axenic cultures (7,29). However, the failure of the uptake of 3H-uracil in our hands needs further investigation since earlier workers had reported its successful uptake by M. tuberculosis (23) and M. leprae (9).
In our hands, M. leprae maintained within macrophages incorporated 10-14-fold higher 3H-adenosine than the same isolates maintained in axenic cultures. Due to the constraints of high numbers of bacilli required in the latter, comparisons were only possible for human-derived bacilli by pooling isolates from several patients. This feature of enhanced metabolism by macro-phage-adapted bacilli was also confirmed with one armadillo strain of M. leprae. Supporting evidence from other workers also indicates the elevated metabolic state of bacilli maintained in host cells (9,29). Studies on lipid as well as carbon metabolism strongly favor the metabolic dependence of the bacilli on the host cell (24,25,30). Moreover, many of the enzymes of the salvage pathway arc highly active in M. leprae, but their de novo synthesis is either suppressed or absent (26,27). The latter defect may make the bacillus dependent on the host cell. It is also possible that the density of bacilli, and the specialized medium used in axenic cultures may suppress metabolic activity due to anaerobic conditions, pH changes, and other unknown requirements of an intracellular organism. However, systematic studies on this may accumulate data toward a better culture medium for the cultivation of M. leprae.
Incorporation of both 3H-hypoxanthinc and 3H-adenosine was inhibited by the an-tileprosy drugs dapsone and rifampin. Whereas the former was effective at 10 ng/ ml, the latter showed significant percent inhibition at the lower concentration of 3 ng/ ml (p < 0.01). These results were similar to our earlier observations on the drug inhibition of 3H-thymidine uptake (11). Although the inhibitory effect of these drugs has been studied in axenic cultures for 3H-hypoxanlhinc (28), as far as we are aware, the effects of drugs on 3H-adcnosine uptake have not been reported. It is evident from these studies that 3H-adenosinc is an effective indicator for the monitoring of M. leprae viability, screening for antileprosy compounds, effectiveness of chemotherapy and diagnosis of drug resistance.
Acknowledgments. K. V. Harshan was supported by the Council of Scientific and Industrial Research, New Delhi. This work was financed by the Indian Council of Medical Research, New Delhi, India.
REFERENCES
1. Ambrose, E. J., Khanolkar, S. R. and Chula-walla, R. G. A rapid test for bacillary resistance to dapsone. Lepr. India 50(1978)131-143.
2. Colston, M. J., Hilston, G. R. F. and Banerjee, D. K. The proportional bacterial test: a method for assessing bactericidal activity of drugs against Mycobacterium leprae in mice. Lepr. Rev. 59( 1978)7-15.
3. Dhople, A. Adenosine triphosphate content of M. leprae from leprosy patients. Int. J. Lepr. 52(1984)183-188.
4. Franzblau, S. G. Oxidation of palmitic acid by Mycobacterium leprae in axenic medium. J. Clin. Microbiol. 26(1988)18-21.
5. Franzblau, S. G., Harris, E. B. and Hastings, R. C. Axenic incorporation of 14C palmitic acid into phenolic glycolipid-I of Mycobacterium leprae. FEMS Microbiol. Lett. 48(1987)407-411.
6. Harris, E. B., Franzblau, S. G. and Hastings, R. C. Inhibition of phenolic glycolipid-I synthesis in extracellular Mycobacterium leprae as an indicator of antimicrobial activity. Int. J. Lepr. 56(1988)588-591.
7. Khanolkar, S. R. and Wheeler, P. R. Purine metabolism in Mycobacterium leprae grown in armadillo liver. FEMS Microbiol. Lett. 20(1983)273-278.
8. Kvach, J. T., Munguia, G. and Strand, S. H. Staining tissue-derived Mycobacterium leprae with fluorescein diacetate and ethidium bromide. Int. J. Lepr. 52(1984)176-182.
9. Mahadevan, P. R., Jagannathan, R., Bhargria, A., Vejare, S. and Agarwal, S. Host-pathogen interaction-new in vitro drug test systems against Mycobacterium leprae-possibilities and limitations. Lepr. Rev. 57(1986)182-200.
10. Mankar, M. V., Jagannathan, R. and Mahadevan, P. R. In vitro drug screening system using membrane alteration in macrophages by Mycobacterium leprae. J. Biosci. 6(1984)709-716.
11. Mittal, A., Seshadri, P. S., Prasad, H. K., Sa-thish, M. and Nath, I. Radiometric macrophage assay for rapid evaluation of anti-leprosy activity of rifampicin. Antimicrob. Agents Chemother. 24(1983)576-585.
12. Nair, I. and Mahadevan, P. R. An in vitro test using cholestrol metabolism of macrophages to determine drug sensitivity and resistance of Mycobacterium leprae. J. Biosc. 6(1984)221-231.
13. Nath, I., Prasad, H. K., Sathish, M. Sreevatsa, Desikan, K. V., Seshadri, P. S. and Iyer, C. G. S. Rapid radiolabcl macrophage culture method for detection of dapsone resistant Mycobacterium leprae. Antimicrob. Agents Chemother. 21(1982)26-32.
14. Prasad, H. K. and Hastings, R. C. Alternate radiolabeled markers for detecting metabolic activity of M. leprae residing in murine macrophages. J. Clin. Microbiol. 21(1985)861-864.
15. Prasad, H. K. and Nath, I. Factors influencing the incorporation of 3H-thymidine in M. leprae residing in differentiated human macrophages. J. Med. Microbiol. 14(1981)279-293.
16. Prasad, H. K., Singh, R. and Nath, I. Radiolabeled M. leprae resident in human macrophage cultures as an indicator of effective immunity in human leprosy. Clin. Exp. Immunol. 49(1982)517-522.
17. Ramasesh, N., Hastings, R. C. and Krahenbuhl, L. Metabolism of Mycobacterium leprae in macrophages. Infect. Immun. 55(1987)1203-1206.
18. Sathish, M. and Nath, I. Uptake of' 3H-thymidinc in M. leprae inoculated mouse macrophage cultures as a rapid indicator of bacillary viability; factors influencing the specificity of the in vitro assay. Int. J. Lepr. 49(1981)187-193.
19. Sathish, M., Rees, R. J. W., Seshadri, P. S. and Nath, I. Comparison of radiometric macrophage assay and the mouse foot pad infection for the evaluation of Mycobacterium leprae sensitivity/ resistance to dapsone. Int. J. Lepr. 53(1985)378-384.
20. Seydel, U., Linder, B. and Dhople, A. M. Results from cation and mass finger print analysis of single cells and from ATP measurements of M. leprae for drug sensitivity testing; a comparison. Int. J. Lepr. 53(1985)365-372.
21. Shepard, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112(1960)445-453.
22. Shepard, C. C. and McRae, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36(1968)78-82.
23. Snider, D. E., Jr., Good, R. C, Kilburn, J. O., Laskowski, L. F., Jr., Lusk, R. H., Marr, J. J., Reggiardo, Z. and Middlebrook, G. Rapid drug-susceptibility testing of Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 123(1981)402-406.
24. Wheeler, P. R. Catabolic pathways of glucose, glycerol and 6-phosphogluconatc in Mycobacterium leprae grown in armadillo tissue. J. Gen. Microbiol. 129(1983)1481-1495.
25. Wheeler, P. R. Metabolism in Mycobacterium leprae. Its relation to other research on M. leprae and to aspects of metabolism in other mycobacteria and intracellular parasites. Int. J. Lepr. 52(1984)208-230.
26. Wheeler, P. R. Biosynthesis and scavenging of purine by pathogenic mycobacteria including Mycobacterium leprae. J. Gen. Microbiol. 133(1987)2999-3011.
27. Wheeler, P. R. Enzymes of purine synthesis and scavenging in pathogenic mycobacteria and their distribution in Mycobacterium leprae. J. Gen. Microbiol. 133(1987)3013-3018.
28. Wheeler, P. R. Measurement of hypoxanthine in purified suspension of Mycobacterium leprae: a suitable method to screen for antilcprosy agents in vitro. J. Med. Microbiol. 25(1988)167-174.
29. Wheeler, P. R. Pyrimidine scavenging by M. leprae. FEMS Microbiol. Lett. 57(1989)179-184.
30. Wheeler, P. R. and Ratledge, C. Use of carbon source for lipid biosynthesis in Mycobacterium leprae: a comparison with other pathogenic mycobacteria. J. Gen. Microbiol. 134(1988)2111-2121.
1. Ph.D., Assistant Research Officer; Department of Biotechnology, All-India Institute ol Medical Sciences, Ansari Nagar, New Delhi 110029, India.
2. Ph.D., Associate Professor; Department of Biotechnology, All-India Institute ol Medical Sciences, Ansari Nagar, New Delhi 110029, India.
3. M.D., M.R.C.Path., M.N.A.M.S., F.A.C.I., F.A.Sc, Professor and Head, Department of Biotechnology, All-India Institute ol Medical Sciences, Ansari Nagar, New Delhi 110029, India.
4. Ph.D., Senior Research Officer, Institute of Pathology; Safdarjang Hospital, New Delhi 110029, India.
5. M.D., Senior Consultant, Dermatology Department, Safdarjang Hospital, New Delhi 110029, India.
6. M.D., District Leprosy Officer, Baroda 390005, India.
Reprint requests to Professor Indira Nath.
Received for publication on 17 July 1989.
Accepted for publication in revised form on 23 February 1990.