- Volume 58 , Number 3
- Page: 534–9
A comparison of monocyte oxidative responses in leprosy patients and healthy subjects as influenced by mycobacterial lipid pretreatment
ABSTRACT
Superoxide anion (O ) release by monocytes f rom leprosy patients in a paired study was lower than that released by monocytes f rom healthy controls. Pretreatment of healthy control monocytes with phenolic glycolipid-l (PGL-I) of Mycobacterium leprae resulted in the release of less O
) release by monocytes f rom leprosy patients in a paired study was lower than that released by monocytes f rom healthy controls. Pretreatment of healthy control monocytes with phenolic glycolipid-l (PGL-I) of Mycobacterium leprae resulted in the release of less O than released by buffer-treated cells or cells pre treated with structurally similar lipids. However, pretreatment of patient monocytes with PGL-I did not affect the O
than released by buffer-treated cells or cells pre treated with structurally similar lipids. However, pretreatment of patient monocytes with PGL-I did not affect the O generation, perhaps because the cells already had a lower capacity to produce O
generation, perhaps because the cells already had a lower capacity to produce O . Upon further examination of the data f rom the patient population, monocytes f rom lepro-matous patients released significantly less O
. Upon further examination of the data f rom the patient population, monocytes f rom lepro-matous patients released significantly less O than cells f rom normal controls, while tuberculoid patient cells released O
than cells f rom normal controls, while tuberculoid patient cells released O in amounts similar to that generated by cells f rom normal controls. In addition, monocytes f rom patients with a high bacterial index had a lower capacity to generate O
in amounts similar to that generated by cells f rom normal controls. In addition, monocytes f rom patients with a high bacterial index had a lower capacity to generate O when compared to cells f rom healthy individuals.
when compared to cells f rom healthy individuals. RÉSUMÉ
La libération d'anions superoxydes (O ) par des monocytes de patients lépreux dans une étude appariée était plus basse que celle de monocytes en provenance de témoins sains. Le traitement préalable des monocytes de témoins sains avec du glycolipide phénolique-1 (GPL-I) de Mycobacterium leprae résulta en la libération de moins d'O
) par des monocytes de patients lépreux dans une étude appariée était plus basse que celle de monocytes en provenance de témoins sains. Le traitement préalable des monocytes de témoins sains avec du glycolipide phénolique-1 (GPL-I) de Mycobacterium leprae résulta en la libération de moins d'O que des cellules traitées par des tampons ou des cellules traitées au préalable avec des lipides de structure similaire. Cependant, le traitement préalable des monocytes des patients avec GPL-I ne modifia pas la production de O
que des cellules traitées par des tampons ou des cellules traitées au préalable avec des lipides de structure similaire. Cependant, le traitement préalable des monocytes des patients avec GPL-I ne modifia pas la production de O , peut-être parce que les cellules avaient déjà une capacité amoindrie de produire de l'oxygène O
, peut-être parce que les cellules avaient déjà une capacité amoindrie de produire de l'oxygène O . Après examen plus approfondi des données en provenance de la population de patients, les monocytes de patients lépromateux libéraient significativement moins d'O
. Après examen plus approfondi des données en provenance de la population de patients, les monocytes de patients lépromateux libéraient significativement moins d'O que les cellules des témoins normaux, tandis que les cellules des patients tuberculoïdcs libéraient des O
que les cellules des témoins normaux, tandis que les cellules des patients tuberculoïdcs libéraient des O en quantités semblables à celles produites par les cellules de témoins normaux. De plus, les monocytes des patients avec un indice bactériologique élevé avaient une capacité moindre de produire des O
en quantités semblables à celles produites par les cellules de témoins normaux. De plus, les monocytes des patients avec un indice bactériologique élevé avaient une capacité moindre de produire des O , en comparaison aux cellules d'individus en bonne santé.
, en comparaison aux cellules d'individus en bonne santé. RESUMEN
La liberación del anión superoxido (O ) por mo-nocitos de pacientes con lepra fue menor que la liberación de O
) por mo-nocitos de pacientes con lepra fue menor que la liberación de O por monocitos de controles sanos. El pretratamiento de los monocitos de controles sanos con el glicolípido fenólico I (PGL-I) de Mycobacterium leprae, dió como resultado la liberación de menos O
por monocitos de controles sanos. El pretratamiento de los monocitos de controles sanos con el glicolípido fenólico I (PGL-I) de Mycobacterium leprae, dió como resultado la liberación de menos O que el liberado por células tratadas con regulador o por células pretratadas con lipidos estructuralmente similares. Sin embargo, el pretratamiento de los monocitos de los pacientes con PGL-I no afectó la generación de O
que el liberado por células tratadas con regulador o por células pretratadas con lipidos estructuralmente similares. Sin embargo, el pretratamiento de los monocitos de los pacientes con PGL-I no afectó la generación de O , quizá porque estas células ya mostraban una menor capacidad para producir O
, quizá porque estas células ya mostraban una menor capacidad para producir O . Comparados con ios controles sanos, los monocitos de los pacientes con lepra tuberculoide produjeron cantidades semejantes de O
. Comparados con ios controles sanos, los monocitos de los pacientes con lepra tuberculoide produjeron cantidades semejantes de O , los monocitos de los pacientes lepro-matosos liberaron cantidades significativamente menores del anión y esta producción de O
, los monocitos de los pacientes lepro-matosos liberaron cantidades significativamente menores del anión y esta producción de O fue todavía menor en los pacientes lepromatosos con índices bacteriológicos altos.
fue todavía menor en los pacientes lepromatosos con índices bacteriológicos altos.
Phenolic glycolipid-I (PGL-I) is unique to Mycobacterium leprae and makes up 2% of the mass of the bacterium (7). It has been detected in the sera, urine, and skin nodules of leprosy patients (2,10,20,21). PGL-I and the related nonglycosylated, nonphenylatcd dimycocerosyl phthiocerol (DIM) arc found in large amounts in experimentally infected armadillos, even in tissues free of M. leprae (3,7,8), indicating that M. leprae releases the lipids into its milieu. The presence of large amounts of PGL-I in tissues infected with M. leprae may be a factor associated with the specific immunological unresponsiveness seen in lepromatous leprosy patients. PGL-I has been shown to induce suppression of mitogenic responses of leprosy patients' lymphocytes in vitro (14,16). We have previously shown (19) that healthy donors' monocytes pretreatcd with PGL-I.release less superoxide anion (O ) when stimulated with M. leprae than nonlipid-treated monocytes. However, monocytes pretreatcd with DIM, mycoside A of M. kansasii, or mycoside B of M. microti, release O
) when stimulated with M. leprae than nonlipid-treated monocytes. However, monocytes pretreatcd with DIM, mycoside A of M. kansasii, or mycoside B of M. microti, release O in quantities comparable to nonlipid-trcated monocytes in response to M. leprae stimulation. In response to other stimuli of the oxidative metabolic burst, such as phorbol myristate acetate (PMA), zymosan, M. bovis BCG, or M. kansasii, monocyte O
in quantities comparable to nonlipid-trcated monocytes in response to M. leprae stimulation. In response to other stimuli of the oxidative metabolic burst, such as phorbol myristate acetate (PMA), zymosan, M. bovis BCG, or M. kansasii, monocyte O release was unaffected by lipid pretreatment.
release was unaffected by lipid pretreatment.
In this study, we examined the effect of PGL-I on leprosy patients' monocytes and assessed the ability of these patients' monocytes to respond oxidatively to various stimuli including M. leprae.
MATERIALS AND METHODS
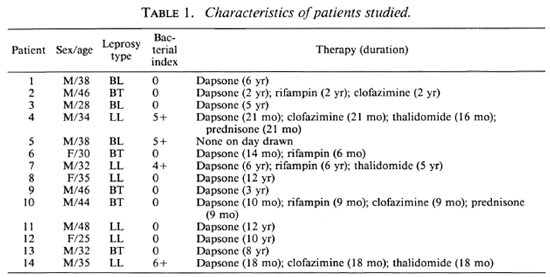
Patients. Informed consent for a blood donation was given by healthy laboratory personnel and University of Illinois students, and by patients over 18 years of age with a confirmed diagnosis of leprosy in the Hansen's Disease Clinic at the University of Illinois at Chicago. Disease classification was according to the criteria of Ridley and Jopling (17). The characteristics of the patient population arc shown in Table 1. To enable us to do paired statistical analysis, blood from a healthy donor within the same age group as the patient was drawn at the same time as the patient. The monocytes of each pair were then analyzed in the same manner.
Monocytes. Peripheral blood was drawn into heparinized (10 IU/ml) syringes. Monocytes were isolated by a modification of the method of Boyum (1). Briefly, the blood was diluted with an equal volume of Hanks' balanced salt solution (HBSS) without calcium or magnesium, layered over Fi-coll-Hypaque (Sigma Chemical Co., St. Louis, Missouri, and Winthrop-Breon, New York, New York, U.S.A.), and centrifuged at 400 x g for 25 min. The mononuclear layer was collected and washed with HBSS without calcium or magnesium. The monocytes were then isolated by elutriation using a Beckman Model J2-21 centrifuge with a JE-6B rotor. The elutriation buffer was HBSS without calcium or magnesium supplemented with EDTA (100 mg/1) and 2% fetal bovine scrum (Hyclone, Logan, Utah, U.S.A.). A flow rate of 21 ml/min and rotor speed of 600 x g were used to exclude the lymphocytes and contaminating platelets and erythrocytes. The flow rate was then increased to 28 ml/min and 200 ml of elutriation buffer was collected to obtain the monocytes. After washing, the cells were re-suspended in RPMI 1640 (Whittaker Bio-products, Inc., Walkersville, Maryland, U.S.A.) medium containing 10% heat-in-activated fetal bovine scrum (Hyclone) and 50 μg/ml gentamicin. The cells were counted, and viability as determined by trypan blue dye exclusion was always greater than 98%. Latex ingestion was used to determine the percentage of phagocytic monocytes (6), and it was consistently greater than 85%.
Lipids. The lipids used in this study, PGL-I, DIM, and mycoside A, were generously supplied by Dr. Patrick J. Brennan (Colorado State University, Fort Collins, Colorado, U.S.A.) through National Institutes of Health Contract no. AI-52582. PGL-I and DIM were purified as previously described (8,9). Mycoside A was isolated from M. kansasii as described previously (4,7). The lipids were each stored in chlo-roform:methanol (2:1), and sonicates were prepared immediately prior to monocyte exposure. A portion of the stock lipids was dried under a stream of nitrogen. HBSS, 1 ml, (Whittaker) was added to the vial and directly sonicated for 30-40 sec with a 3-mm probe of a Fisher Sonic Dismembra-tor Model 300 at 70% relative output.
Culture conditions. Buffer or sonicates of cither PGL-I, DIM, or mycoside A were added to monocytes in teflon vials (Savillex, Minnetonka, Minnesota, U.S.A.) at 100 μg lipid/2 x 106 cells. The vials were tumbled end over end at 12 rotations/min for 2 hr at 37ºC. The cells were then transferred to centrifuge tubes and washed. We have previously determined that under these conditions an average of 15 μg PGL-I is taken up by the monocytes as determined by dot-blot analysis following the extraction of the PGL-I from the monocytes (19). We have also shown that DIM and mycoside A were taken up in comparable amounts by the monocytes (19). The cells were counted and their viability, after treatment with the various lipids, was greater than 98%. The cells were placed in 96-well, flat-bottom microliter plates (Corning Inc., Corning, New York, U.S.A.) at 2 x 105 cells/well in a 0.2 ml volume of media and incubated overnight at 37ºC in 5% CO2 in humidified air. After washing the wells, O release by the adherent monocytes was determined.
release by the adherent monocytes was determined.
Superoxide anion assay. release was determined by measuring the superoxide dismutase (SOD)-inhibitable cytochrome c (Sigma) reduction as described previously (5,15). Briefly, cytochrome c and stimulus in HBSS without phenol red were added to triplicate wells which were blanked against triplicate wells containing SOD in addition to the cytochrome c and stimulus. The ab-sorbancc was measured at 550 nm using an EIA Reader (Biotek, Burlington, Vermont, U.S.A.) immediately after the addition of reaction mixtures (0 time) and 150 min later. The amount of O reported is that released by the cells in the 150 min time interval. We chose to read the plates after 150 min instead of the conventional 90 min because we had previously shown (5) that M. leprae is a weak and slow stimulus of the metabolic burst of monocytes. Between readings, the plates were incubated at 37ºC on a microliter plate shaker (Dynatech Laboratories, Alexandria, Virginia, U.S.A.) to facilitate the interaction of the particulate stimuli with the monocytes. The stimuli used in the assay included opsonized zymosan (1:1 v/v with fresh scrum at 37ºC for 20 min), irradiated M. leprae, and M. kansasii (all at 50:1 particle-to-monocyte ratio) and PMA (Sigma; 500 ng/ml). The preparation of the bacteria for use in the O
reported is that released by the cells in the 150 min time interval. We chose to read the plates after 150 min instead of the conventional 90 min because we had previously shown (5) that M. leprae is a weak and slow stimulus of the metabolic burst of monocytes. Between readings, the plates were incubated at 37ºC on a microliter plate shaker (Dynatech Laboratories, Alexandria, Virginia, U.S.A.) to facilitate the interaction of the particulate stimuli with the monocytes. The stimuli used in the assay included opsonized zymosan (1:1 v/v with fresh scrum at 37ºC for 20 min), irradiated M. leprae, and M. kansasii (all at 50:1 particle-to-monocyte ratio) and PMA (Sigma; 500 ng/ml). The preparation of the bacteria for use in the O assay has been previously described in detail (5,6). Protein determinations were performed on the washed adherent cells by the Lowry, et al. method (11) with bovine serum albumin (Sigma) used for the standard curve. The amount of cell protein did not differ significantly between the lipid-treated and the nonlipid-treatcd control groups. The amount of released by nonstimulated monocytes was always substantially lower than that released by stimulated cells regardless of the origin of the cells.
assay has been previously described in detail (5,6). Protein determinations were performed on the washed adherent cells by the Lowry, et al. method (11) with bovine serum albumin (Sigma) used for the standard curve. The amount of cell protein did not differ significantly between the lipid-treated and the nonlipid-treatcd control groups. The amount of released by nonstimulated monocytes was always substantially lower than that released by stimulated cells regardless of the origin of the cells.
Endotoxin contamination. Endotoxin was not detected in the lipid preparations, buffers or media as determined by a quantitative chromogenic limulus amebocyte lysate assay (Whittaker) with a sensitivity of 10 pg/ml.
Statistics. Differences between control and experimental groups were evaluated for statistical significance by Student's t test.
RESULTS
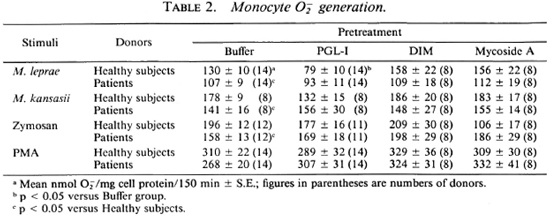
As shown in Table 2, buffer-pretreated monocytes from leprosy patients released significantly (p < 0.05) less O than monocytes from healthy donors in response to the particulate stimuli, M. leprae, M. kansasii, and zymosan. However, patient monocytes pretreated with PGL-I, DIM, or mycosidc A did not differ from monocytes of the healthy donors in the amount of O
than monocytes from healthy donors in response to the particulate stimuli, M. leprae, M. kansasii, and zymosan. However, patient monocytes pretreated with PGL-I, DIM, or mycosidc A did not differ from monocytes of the healthy donors in the amount of O released (Table 2). We had previously shown (19), and it was observed in this study, that healthy donors' monocytes prctreated with PGL-I released less O
released (Table 2). We had previously shown (19), and it was observed in this study, that healthy donors' monocytes prctreated with PGL-I released less O than buffer-treated cells when the stimulus was M. leprae (Table 2). Interestingly, monocytes from tuberculoid patients responded to M. leprae with significantly (p < 0.05) less O
than buffer-treated cells when the stimulus was M. leprae (Table 2). Interestingly, monocytes from tuberculoid patients responded to M. leprae with significantly (p < 0.05) less O release when pretreated with PGL-I than when buffer-treated (71 ± 16 vs 121 ± 14 nmole O
release when pretreated with PGL-I than when buffer-treated (71 ± 16 vs 121 ± 14 nmole O / mg cell protein/150 min ± S.E., respectively; N = 5). In contrast, lepromatous patients' monocytes stimulated with M. leprae released similar amounts of whether or not they had been pretreated with PGL-I (98 ± 11 vs 100 ± 20 nmole O
/ mg cell protein/150 min ± S.E., respectively; N = 5). In contrast, lepromatous patients' monocytes stimulated with M. leprae released similar amounts of whether or not they had been pretreated with PGL-I (98 ± 11 vs 100 ± 20 nmole O /mg cell protein/150 min ± S.E.; N = 9).
/mg cell protein/150 min ± S.E.; N = 9).
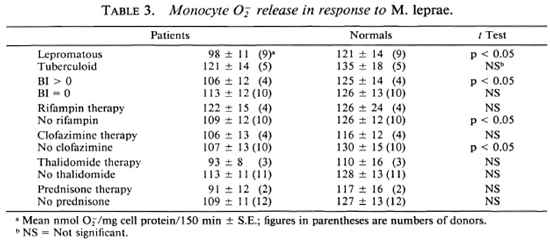
When the data from the patient population were further analyzed, it was observed that the lepromatous patients' monocytes not treated with any lipids released significantly (p < 0.05) less O than monocytes from healthy subjects in response to M. leprae; whereas monocytes from the tuberculoid patients generated amounts of O
than monocytes from healthy subjects in response to M. leprae; whereas monocytes from the tuberculoid patients generated amounts of O similar to those generated by monocytes from the healthy donors (Table 3). Cells from patients with a high bacterial index released significantly (p < 0.05) less O
similar to those generated by monocytes from the healthy donors (Table 3). Cells from patients with a high bacterial index released significantly (p < 0.05) less O in response to M. leprae than did the cells from the corresponding healthy individuals. Monocytes from patients with a negative bacterial index released similar amounts of Of when challenged with M. leprae as did cells from the paired healthy subjects (Table 3). O
in response to M. leprae than did the cells from the corresponding healthy individuals. Monocytes from patients with a negative bacterial index released similar amounts of Of when challenged with M. leprae as did cells from the paired healthy subjects (Table 3). O release by monocytes from patients on the various therapies (dapsone, rifampin, clofazimine, thalidomide, or prednisone) did not differ from that released by monocytes from healthy donors (Table 3). Monocytes from patients not receiving rifampin or clofazimine released significantly less O
release by monocytes from patients on the various therapies (dapsone, rifampin, clofazimine, thalidomide, or prednisone) did not differ from that released by monocytes from healthy donors (Table 3). Monocytes from patients not receiving rifampin or clofazimine released significantly less O than did monocytes from the healthy donors paired with those patients. Although the number of donors studied is small, and we cannot exclude a selection bias in the type of patients receiving these drugs, these data suggest that rifampin or clofazimine therapy can help patients in terms of their monocyte oxidative responses.
than did monocytes from the healthy donors paired with those patients. Although the number of donors studied is small, and we cannot exclude a selection bias in the type of patients receiving these drugs, these data suggest that rifampin or clofazimine therapy can help patients in terms of their monocyte oxidative responses.
The data were analyzed by a paired Student's t test, where appropriate, since a healthy donor was drawn the same day as each patient and the monocytes from both donors were treated in a similar manner.
DISCUSSION
Monocytes from leprosy patients released less O than monocytes from healthy subjects when stimulated with M. leprae, M. kansasii, or zymosan. There was no difference in the Of release when the stimulus was PMA, perhaps because PMA is such a potent stimulus of the oxidative burst of phagocytes. We had previously shown (19) that PGL-I-pretreated monocytes from healthy donors, when stimulated with M. leprae, released less O
than monocytes from healthy subjects when stimulated with M. leprae, M. kansasii, or zymosan. There was no difference in the Of release when the stimulus was PMA, perhaps because PMA is such a potent stimulus of the oxidative burst of phagocytes. We had previously shown (19) that PGL-I-pretreated monocytes from healthy donors, when stimulated with M. leprae, released less O than did buffer-pre-treated cells or cells pretreated with lipids structurally similar to PGL-I. We suspect that we did not observe depressed Of release by PGL-I-pretreated patient monocytes compared to buffer-pretreated patient monocytes because the latter group of cells released less O
than did buffer-pre-treated cells or cells pretreated with lipids structurally similar to PGL-I. We suspect that we did not observe depressed Of release by PGL-I-pretreated patient monocytes compared to buffer-pretreated patient monocytes because the latter group of cells released less O than buffer-pretreated monocytes from the healthy donors. On further analysis of the data, the following observations were made. Monocytes from lepromatous patients released less O
than buffer-pretreated monocytes from the healthy donors. On further analysis of the data, the following observations were made. Monocytes from lepromatous patients released less O than monocytes from tuberculoid patients or from healthy donors. The monocytes from patients with a high bacterial index had depressed O
than monocytes from tuberculoid patients or from healthy donors. The monocytes from patients with a high bacterial index had depressed O production compared to monocytes from healthy donors. O
production compared to monocytes from healthy donors. O release by monocytes from patients with a negative bacterial index did not differ from that released by monocytes from healthy subjects. In addition, monocytes from patients on rifampin or clofazimine therapy did not differ from monocytes of healthy subjects in their oxidative responses. It appears that therapy may restore the ability of leprosy patients' monocytes to respond oxidatively.
release by monocytes from patients with a negative bacterial index did not differ from that released by monocytes from healthy subjects. In addition, monocytes from patients on rifampin or clofazimine therapy did not differ from monocytes of healthy subjects in their oxidative responses. It appears that therapy may restore the ability of leprosy patients' monocytes to respond oxidatively.
The results of previous studies measuring oxidative product release by leprosy patients' monocytes are not in clear agreement. The spontaneous and stimulated ni-troblue tetrazolium (NBT) reduction as a measure of monocyte O release has been reported to be elevated in leprosy patients when compared to healthy individuals (13). In contrast, monocyte O
release has been reported to be elevated in leprosy patients when compared to healthy individuals (13). In contrast, monocyte O release, as measured by cytochrome c reduction, in response to M. leprae and PMA was reportedly similar whether the cells were from leprosy patients or from healthy subjects (18). Most recently, it was reported that mono-cyte-derived macrophages from leprosy patients were unable to produce O
release, as measured by cytochrome c reduction, in response to M. leprae and PMA was reportedly similar whether the cells were from leprosy patients or from healthy subjects (18). Most recently, it was reported that mono-cyte-derived macrophages from leprosy patients were unable to produce O (as measured by N13T) in response to live M. leprae, while heat-killed M. leprae induced release by cells from tuberculoid and bacte-riologically negative LL patients but not bacteriologically positive LL patients (12).
(as measured by N13T) in response to live M. leprae, while heat-killed M. leprae induced release by cells from tuberculoid and bacte-riologically negative LL patients but not bacteriologically positive LL patients (12).
None of the studies mentioned above have been paired studies. Ours took into account assay variability, and we performed the experiments on one patient's and one healthy donor's monocytes at the same time. The cytochrome c reduction method wc used is the more generally used method to determine O release. In addition, the microas-say we utilized is ideal for continuous measurements of O
release. In addition, the microas-say we utilized is ideal for continuous measurements of O generation, and allows for monitoring simultaneous replicates for each stimulus desired. Moreover, to better standardize the O
generation, and allows for monitoring simultaneous replicates for each stimulus desired. Moreover, to better standardize the O assay and to assure that the number of cells remaining in the wells were the same for the various donor populations, wc performed protein determinations on the monocyte-containing wells. We have reported our data as nmol O
assay and to assure that the number of cells remaining in the wells were the same for the various donor populations, wc performed protein determinations on the monocyte-containing wells. We have reported our data as nmol O per mg cell protein per unit of time. None of the above studies took the protein content of the wells into account. The results of Mariola and Mahadevan (12) are in closest agreement with our data. However, the M. leprae used in our studies were irradiated, our assay measured monocyte O
per mg cell protein per unit of time. None of the above studies took the protein content of the wells into account. The results of Mariola and Mahadevan (12) are in closest agreement with our data. However, the M. leprae used in our studies were irradiated, our assay measured monocyte O release by the cytochrome c reduction method, and ours was a paired study.
release by the cytochrome c reduction method, and ours was a paired study.
Monocytes/macrophages arc preferential host cells for parasitization by M. leprae. Modulation of oxidative responses, such as O release of these cells, may contribute to the ability of M. leprae to survive intracellulary. A better understanding of the interaction of M. leprae with these cells is necessary to further our understanding of the pathogenesis of leprosy.
release of these cells, may contribute to the ability of M. leprae to survive intracellulary. A better understanding of the interaction of M. leprae with these cells is necessary to further our understanding of the pathogenesis of leprosy.
Acknowledgments. This work was supported by the University of Illinois Earl M. Bane Trust Fund and the Veterans Administration Merit Review Grant MRSI-034.
We thank Dr. Patrick J. Brennan for supplying M. leprae, PGL-I, DIM, and mycoside A. We are grateful to Arlene Slajchert for coordinating the patient studies and to Vijai Moses for assistance with the statistical analysis. We thank Harold Amirault forassistance with phlebotomy and Bea Wozniak for skillful secretarial assistance.
REFERENCES
1. Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21Suppl.(1968)77-89.
2. Cho, S.-N., Hunter, S. W., Gelber, R. H., Rea, T. H. and Brennan, P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153(1986)560-569.
3. Draper, P., Payne, S. N., Dobson, G. and Minnikin, D. E. Isolation of a characteristic phthiocerol dimycocerosate from Mycobacterium leprae. J. Gen. Microbiol. 129(1983)859-863.
4. Gastambide-Odier, M. and Sarda, P. Contribution a l'étude de la structure de la biosynthesc de glycolipides spécifiques isolés de mycobacte-rics: les mycosides A et B. Pneumologie 142(1970)241-255.
5. Holzer, T. J., Kizlaitis, K., Vachula, M., Weaver, C. W. and Andersen, B. R. Human phagocytic cell responses to Mycobacterium leprae and Mycobacterium bovis bacillus Calmette Guérin: an in vitro comparison of leprosy vaccine components. J. Immunol. 141(1988)1701-1708.
6. Holzer, T. J., Nelson, K. E., Schauf, V., Crispen, R. G. and Andersen, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51(1986)514-520.
7. Hunter, S. W. and Brennan, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
8. Hunter, S. W. and Brennan, P. J. Further specific extracellular phenolic glycolipid antigens and a related diacylphthiocerol from Mycobacterium leprae. J. Biol. Chem. 258(1983)7556-7562.
9. Hunter, S. W., Fujiwara, T. and Brennan, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
10. Kaldany, R. R., Maasiio, K., Ohman, R., Reitz, V. D., Britton, S. and Lefford, M. J. Methods for detection of a specific Mycobacterium leprae antigen in the urine of leprosy patients. Scand. J. Immunol. 25(1987)37-43.
11. Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
12. Mariola, J. and Mahadevan, P. R. Superoxide production from macrophages of leprosy patients after stimulation with Mycobacterium leprae. J. Biosci. 12(1987)273-279.
13. Masuda, A. and Scheinberg, M. A. Peripheral blood monocyte function in leprosy patients. Int. J. Lepr. 48(1980)254-259.
14. Mehra, V., Brennan, P. J., Rada, E., Convit, J. and Bloom, B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308(1984)194-196.
15. Pick, E. and Mizel, D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J. Immunol. Meth. 46(1981)211-226.
16. Prasad, H. K., Misiira, R. S. and Nath, I. Phenolic glycolipid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin a responses unrelated to leprosy type. J. Exp. Med. 165(1987)239-244.
17. Ridley, D. S. and Jopling, W. H. A classification of leprosy for research purposes. Lepr. Rev. 33(1962)119-128.
18. Sharp, A. K. and Banerjee, D. K. Hydrogen peroxide and superoxide anion production by peripheral blood monocytes in leprosy. Clin. Exp. Immunol. 60(1985)203-206.
19. Vachula, M., Holzer, T. J. and Andersen, B. R. Suppression of monocyte oxidative responses by phenolic glycolipid I of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.
20. Vemuri, N., Khandke, L., Mahadevan, P. R., Hunter, S. W. and Brennan, P. J. Isolation of phenolic glycolipid from human lepromatous nodules. Int. J. Lepr. 53(1985)487-489.
21. Young, D. B. Detection of mycobacterial lipids in skin biopsies from leprosy patients. Int. J. Lepr. 49(1981)198-204.
1. Ph.D., Departments of Medicine, Microbiology/Immunology, University of Illinois at Chicago, Chicago, Illinois.
2. M.D., Department of Dermatology, University of Illinois at Chicago, Chicago, Illinois.
3. M.D., Departments of Medicine, Microbiology/Immunology, University of Illinois at Chicago, and West Side Veterans Administration Medical Center, Chicago, Illinois, U.S.A.
Present addresses: Dr. M. Vachula, Baxter Healthcare, WG2-2S, Route 120 and Wilson Road, Round Lake, Illinois 60073, U.S.A. Dr. S. Worobec, Dermatology, Ortho Pharmaceutical Corporation, Route 202, P.O. Box 300, Raritan, New Jersey 08869-0602, U.S.A.
Reprint requests to: Dr. Burton R. Andersen, Chief, Section of Infectious Diseases, M/P 151, WSVA, 820 S. Damen Avenue, Chicago, Illinois 60612, U.S.A.
Received for publication on 17 July 1989.
Accepted for publication in revised form on 14 February 1990.