- Volume 58 , Number 3
- Page: 540–7
Antigenic protein f rom Mycobacterium leprae released in macrophages in vitro as indicator of viability of bacteria
ABSTRACT
Peritoneal macrophages f rom random-bred, Swiss white mice, when cultured and infected with Mycobacterium leprae for 24 hours, are able to show the presence of an-tigen(s) with binding affinity to antibodies present in the sera of bacteriologically positive, lepromatous leprosy patients. Such antibodies are not seen in sera f rom normal and healthy persons, tuberculoid leprosy patients, or long-term-treated, bacteriologically negative, lcpromatous leprosy patients. The production of the antigen(s) is blocked by the anti-M. leprae drug rifampin. Other mycobacteria when incubated with macrophages f rom mice show very little antigens in the lysate but the antigens have an equal affinity for antibodies in sera f rom both normal individuals and lepromatous patients. Only the lysates f rom macrophages exposed to live M. leprae could discriminate and could exhibit differential binding to sera f rom leprosy patients compared to sera f rom normal individuals. This antigcn(s) does not have any binding ability to the monoclonal antibodies available to the antigens of M. leprae identified at present and shown to be specific to M. leprae. This indicates a separate identity of this product which has potential for further exploitation in exploring host-pathogen interactions related specifically to the leprosy infection and the tolerance of M. leprae inside cells.RÉSUMÉ
Les macrophages péritonéaux de souris blanches Swiss d'élevage, mis en culture et infectés par Mycobacterium leprae pendant 24 heures, peuvent montrer la présence d'antigènes ayant une allinité pour des anticorps présents dans le sérum de lépreux lépromateux ayant une bactériologie positive. De tels anticorps ne sont pas observés dans le sérum de personnes normales en bonne santé, les patients lépreux tuberculoïdes, ou les lépreux lépromateux traités depuis longtemps et bactériologiquement négatifs. La production d'anti-gène(s) est bloquée par la rifampicine. Les autres my-cobactéries, incubées avec des macrophages de souris, montrent très peu d'antigènes dans le lysat, mais les antigènes ont une affinité identique pour les anticorps sériques provenant d'individus normaux et de lépreux lépromateux. Seuls les lysats de macrophages exposés à des M. leprae vivants pouvait faire la distinction et présenter une affinité différente pour les sérums de patients lépreux et ceux d'individus normaux. Cet anti-gènc(s) n'a aucune capacité de se lier aux anticorps monoclonaux disponibles vis-à-vis des antigènes de M. leprae identifiés à présent, et dont la spécificité pour M. leprae a été démontrée. Ceci indique que ce produit a une identité propre, et qu'il mérite d'être utilisé plus avant pour explorer les interactions hotc-pathogène liées spécifiquement à l'infection lépreuse, et la tolérance de M. leprae à l'intérieur des cellules.RESUMEN
Los macrófagos peritoneales de ratones Swiss-white cultivados e infectados con Mycobacterium leprae por 24 horas, son capaces de mostrar la presencia de antí-genos que reaccionan con anticuerpos presentes en los sueros de pacientes con lepra lepromatosa bacteriológicamente positivos. Tales anticuerpos no se encuentran en los sueros de personas sanas, de pacientes con lepra tubereuloide, o de pacientes lepromatosos con mucho tiempo de tratamiento y bacteriológicamente negativos. La producción de los antígenos se bloquea por la droga antileprosa rifampina. Cuando se incuban otras micobacterias con macrófagos de ratón, se encuentran muy pocos antígenos en el lisado celular que tienen una afinidad igual por anticuerpos presentes tanto en los sueros de individuos normales como en los sueros de pacientes lepromatosos. Sólo los lisados de los macrófagos expuestos al M. leprae vivo pudieron discriminar, por su enlazamiento diferencial, a los sueros de los pacientes lepromatosos de aquellos de los individuos normales. Estos antigenos no tienen reactividad con los anticuerpos monoclonales existentes a la fecha que identifican antígenos del M. leprae. Esto indica una diferente identidad de estos productos y sugiere su utilidad potencial en el estudio de las interacciones huésped-parásito relacionadas específicamente con la infección y la tolerancia del M. leprae en el interior de las células.Macrophages are the cells that primarily harbor Mycobacterium leprae, the causative organism of leprosy. Macrophages isolated from the peripheral blood of humans and the peritoneal cavity of mice have been shown to phagocytose M. leprae in vitro. The ability of macrophages to kill M. leprae is generally believed to be poor in leprosy patients, and this is especially so in the cells from lepromatous leprosy patients (9). To understand further the host-pathogen interaction in leprosy, we have studied the changes introduced in the host cells, macrophages from humans and mice, by live M. leprae in relation to both internal metabolism and membrane structures (1,4-6,8,12). It was clear from these observations that in both the leprosy patients and susceptible mice, such as the Swiss white strain, the macrophages were modified both in their structure and function by the presence of live M. leprae.
While M. leprae were phagocytosed by macrophages in vitro, the metabolic ability of M. leprae inside such macrophages has been demonstrated primarily by the uptake of precursor molecules, such as thymidine, uracil and hypoxanthine (3,11,14,15). But actual multiplication inside the cultured macrophages has not been demonstrated.
In this report, we describe the presence of antigen(s) in M. leprae-infected, in vitro cultures of mouse macrophages by the affinity of these antigen(s) for antibodies present in lepromatous leprosy patients as demonstrated by an enzyme-linked immunosorbent assay (ELISA). We further show that these antigens are products of the metabolism of M. leprae and of the viable bacilli inside the cells.
MATERIALS AND METHODS
M. leprae. M. leprae were obtained from tissues such as the spleen and liver from an infected armadillo, and were kindly provided by Dr. E. Storrs, Melbourne, Florida, U.S.A. The tissues were collected under aseptic conditions and transported on dry ice to Bombay, India, within a period of 1 week. The tissues were stored at - 90ºC, and pieces were taken out as needed.
Such tissues from the M. leprae-infected armadillo were processed by washing the pieces in normal saline and chopping them in a sterile petri dish. They were homogenized with a hand homogenizer, and cen-trifuged at 276 x g x 10 min to remove tissue debris. The supernatant was further centrifuged at 3071 x g x 30 min, and the pellet suspended in saline. A smear was prepared, stained for acid-fast bacilli (AFB), and quantitated. The bacilli were identified as acid-fast stainable by the Ziehl-Neelsen method, and were free from contaminating fast-growing microbes.
Macrophage cultures. Following the injection of 5 ml of Eagle's minimal essential medium (MEM) + 20% heat-inactivated human serum into the peritoneal cavity of random-bred Swiss white mice, the peritoneal fluid was withdrawn and distributed in sterile 35-mm petri plates (Falcon). The cultures were incubated at 37ºC in a 5% CO2 atmosphere, and the medium of each culture was changed every 24 hr to remove nonadherent cells. After 3 days of culture, esterase-positive phagocytic macrophages were obtained with very few other cell types. M. leprae (30 x 106/petri dish) were added to these macrophage cultures, incubated at 37ºC for 24 hr, and the supernatant removed to delete excess M. leprae. Control experiments showed that there were about 1 x 106 macrophages in each dish. By the addition of 30 x 106 M. leprae, we get a ratio of 30:1 M. leprae to host cell in the culture. In actual experiments, the ratio was 25:1 to 40:1; phagocytosis could vary the number of M. leprae per macrophage. The adherent cells that had phagocytosed M. leprae, after washing with MEM twice, were scraped offand lysed by ten cycles of freeze-thawing. The resulting suspension was cen-trifuged at 21,000 x g x 30 min, and the supernatant was filtered through a 0.22 μm Millipore membrane to obtain particle-free, sterile cell lysate. This lysate was the source of the antigens used for testing with the sera from patients. The proteins in the lysate were quantitated by the method of Lowry, et al. (7).
ELISA. These lysates were used as the source of antigen(s) and coated on 96-well polystyrene plates (Laxboro, Pune, India). An ELISA using human sera for primary antibodies and peroxidase-conjugated antihuman immunoglobulin (IgG) (Miles Laboratories, Slough, U.K.) was carried out as described for M. leprae phenolic glyco-lipid (2,16)- The binding of antibodies in the sera to the antigens in the lysate of the macrophages was indicated by the color development during the ELISA, determined as optical density (OD) (492 nm) in the Multiscan ELISA reader (Flow Laboratories, U.K.). The OD intensity indicated the concentration of antibody in the sera to the antigen(s) under test.
Variations in the experiments were those using macrophage lysate prepared after incubating either heat-killed M. leprae, irradiated M. leprae, or live M. leprae in the presence of 5 μg/ml of rifampin (Sigma Chemical Co., U.K.) for 72 hr or with 10 μg/ml of cycloheximide (Sigma, U.K.) for 24 hr. The cells were also exposed to a number of cultivable mycobacteria (30 x 106), and lysates were prepared as described earlier to compare their behavior with sera from leprosy patients.
The sera were prepared from 5 ml of blood obtained from bacillary-positive, untreated or short-term (6 months) treated, lepromatous leprosy patients (B + LL); bacillary-negative, long-term (> 4 years) treated, lepromatous leprosy patients (B-LL); and paucibacillary patients (T). The blood (5 ml) was donated voluntarily by patients while attending the outpatient clinics in Bombay. Blood was also collected from normal healthy individuals (N) who were exposed in varying degrees to M. leprae in the city of Bombay. These sera were kept frozen at -20ºC until used.
For determining the optimum incubation time, macrophage cultures were infected with M. leprae and the cultures were terminated at various time intervals from 24144 hr. The lysates obtained were tested for their ability to bind to serum antibodies using the ELISA.
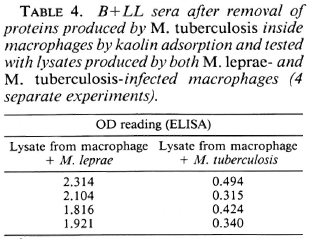
Kaolin (Sigma, U.K.) agglutination, based on a method described by Takahashi (13), was used to test for competition in the binding ability of serum to antigens induced especially in the presence of M. tuberculosis. Acid-washed kaolin powder was used: one part standard kaolin and two parts of the lysate antigen (10 μg) were agitated together and incubated for 30 min at 37ºC. Serial dilutions of serum (B + LL) were made at 1:100 and 1:500 such that the total volume was 500 μl. To this was added sensitized kaolin, and the tubes were incubated at 37ºC for 30 min, and then centrifuged at 1500 x g x 10 min. The supernatant (the adsorbed serum) was then tested by ELISA against another fresh sample of lysate antigen. The lysate prepared by incubating macrophages with both M. tuberculosis and M. leprae was coated on kaolin and was used to adsorb B+LL sera. The adsorbed sera was then tested against fresh lysate antigen made either with M. leprae or M. tuberculosis, and the effect of such modification on the OD level was recorded.
Monoclonal antibodies were obtained from the WHO IMMLEP group and also from the London laboratory of Dr. J. Ivanyi.
Statistical significance was calculated using Student's t test. Average values arc expressed along with standard deviations (S.D.).
RESULTS
The binding ability of sera from various types of individuals to the lysate antigen produced in the presence of M. leprae is depicted as the OD recorded after ELISA in Figure 1 A. It is clear that only those sera from bacillary-positive lepromatous leprosy patients (B + LL) show high binding ability to the antigen(s). The average OD reading with B + LL sera was quite high compared to that obtained from the other types of sera (p < 0.0005). It is also clear that the presence of M. leprae and bacteriological positivity was necessary in the patients for the maintenance of the antibody in the scrum, since long-term-treated, bacillary-negative, lepromatous patients (B -LL) showed essentially no antibodies in their sera (OD < 0.6).
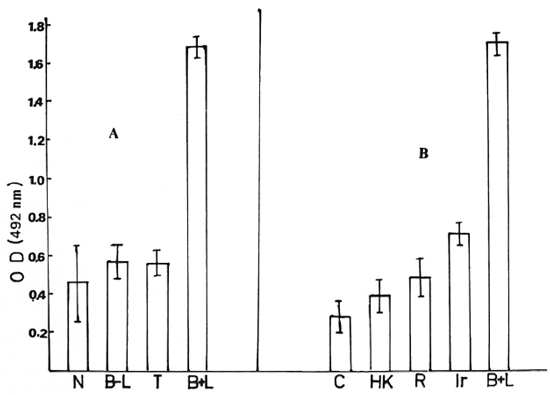
Fig. 1A. ELISA positivity between antigen(s) in the lysate and sera from various categories of individuals: N = sera from 20 normal individuals; B-L = 10 bacteriologically negative lepromatous leprosy patients; T = 10 tuberculoid leprosy patients; B + L = 9 bacteriologically positive lepromatous leprosy patients. Each sample was done in triplicate. B + L bar indicates mean ± 1 S.D., others indicate mean ± 3 S.D. to reveal significant differences between them and B + L. Antigen (protein) concentration was 5 μg; serum dilution, 1:500.
Fig. 1B. ELISA positivity between antigen prepared with various modifications and tested with sera from bacteriologically positive lepromatous leprosy patients. C = macrophage lysate (no addition); HK = with heat-killed M. leprae added; R = in presence of M. leprae and rifampin; Ir = with irradiated M. leprae; B + L = with live M. leprae and serum from bacteriologically positive lepromatous leprosy patient. Values are mean ± S.D. Antigen (protein) concentration was 5 μg; serum dilution, 1:500.
The variability of the antigen level induced by M. leprae inside the cells could be due to the variable percentage of viable bacteria in the samples of M. leprae used (28%-42%) as determined by the fluorescein di-acctate (FDA) staining method. On the average, 6-10 x 106 M. leprae were phagocytosed by 106 macrophages in all of the experiments. The bacilli were counted after being lysed out from the macrophages after the determination of viability. By using lysate prepared after infecting the peritoneal macrophages with M. leprae in separate experiments, one could get large numbers of separate antigenic samples. The data presented in Figure 1B show the binding ability of various antigenic samples obtained using one or more serum samples from B + LL patients.
Neither heat-killed M. leprae nor irradiated M. leprae were able to induce any an-tigen(s) inside the macrophages (Fig. 1B). If 5 μg/ml of rifampin was added to the macrophage cultures 72 hr earlier and was present at the time of M. leprae entry and during incubation, no antigen(s) were produced in the macrophage lysate.
Table 1 shows that the ELISA positivity (OD) was proportional to the antibody concentration and also proportionate to the relative concentration of the lysate. The concentration of the lysate as protein was optimum at 1 μg/well, and the antibody at a serum dilution of 1:500 gave good discrimination between the positive sera (B + LL) and negative sera (N). Protease treatment resulted in a loss of antigenicity (data not shown).
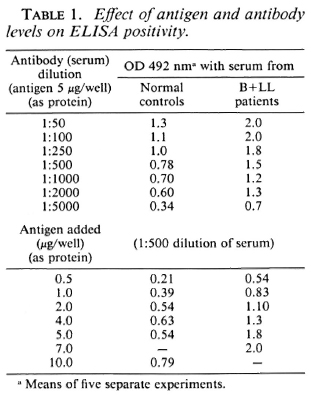
Figure 2 shows the time course of the appearance of the antigen(s) inside the macrophages after the entry of M. leprae. The rate was slow at the beginning but increased rapidly between 20-24 hr of incubation, was maximum at 24 hr, remained steady until 48 hr, and decreased by 72 hr. There was even less demonstrable antigen by 144 hr of incubation (Fig. 2).
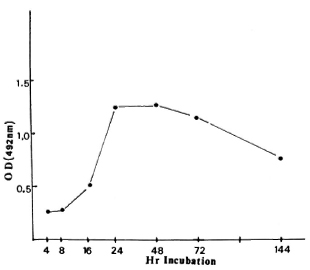
Fig. 2. Antigen concentration in the ELISA lysate as expressed by optical density (OD) in relation to time of incubation of live M. leprae inside macrophages.
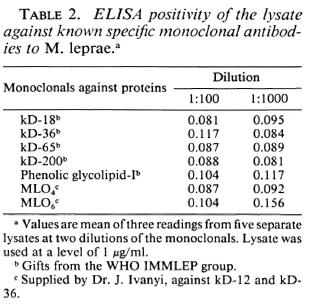
The macrophage lysate was tested for binding to the monoclonal antibodies known to be specific for other M. leprae antigens. None of them gave a positive binding to the lysate antigen obtained in these experiments, even at a 1:100 dilution of the monoclonal antibodies (Table 2). No competition assay was carried out. Hence, the macrophage lysate could contain a different protein antigen from those previously described and could be being recorded for the first time now using the sera from leprosy patients.
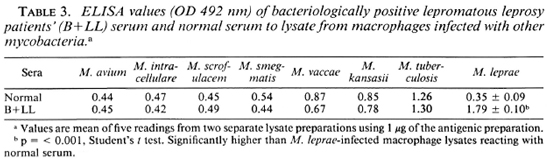
Preliminary experiments showed that rabbit polyclonal antisera against the macrophage lysate could recognize one faint band of M. leprae by Western blot (data not shown), but more detailed studies arc needed. Lysate produced by using macrophage cultures exposed to other mycobacteria showed some ELISA positivity with sera from both lepromatous leprosy patients and normal healthy individuals. These lysates could not discriminate between normal sera and B + LL sera. More specifically, these lysates showed no higher affinity for B + LL sera than for normal sera, while this was readily demonstrable with the M. leprae-induced lysate (Table 3). Lysates obtained by incubating macrophages with M. tuberculosis gave a positive reaction with sera from B + LL patients, with an equal binding ability to antibodies in normal serum.
B+LL sera adsorbed with kaolin particles coated with lysate proteins produced by M. tuberculosis-infected macrophages no longer bound to lysatcs from M. tuberculosis-infected macrophages but still bound to lysatcs from M. leprae-infected macrophages (Table 4). Further, if adsorption of the serum antibody was done with kaolin particles coated with M. leprae-induced lysate antigens, the adsorbed serum showed poor affinity (low OD) with M. leprae-induced lysate as compared to M. tuberculosis-induced lysate (Table 5).
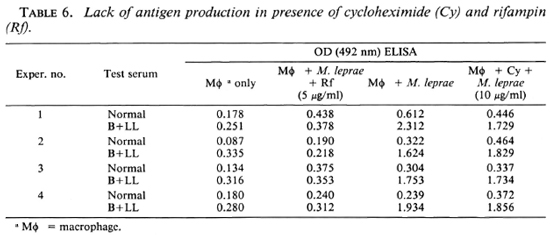
Antigen production was tested in the presence of cyclohcximide, a protein synthesis inhibitor of eukaryotic cells. Cyclohcximide did not inhibit the production of the lysate antigen, suggesting that it is produced by M. leprae in the macrophage (Table 6).
DISCUSSION
The observations show that M. leprae-infected macrophages produce antigen(s) that have an affinity for immunoglobulins present in the sera of bacteriologically positive lepromatous leprosy patients. Such antigens are not produced in the presence of dead M. leprae nor in the presence of an antileprosy drug such as rifampin. No other sera tested have immunoglobulins that have significant binding ability for these antigen(s).
The lysate produced from mouse macrophages on incubation with live M. leprae binds with the sera from bacteriologically positive lepromatous leprosy patients much better (OD 1.7) than it binds with sera from normal healthy individuals (OD 0.45). The antibodies recognized by the M. leprae-infected macrophage lysate antigens appear to be primarily of the IgG and not IgM type (data not shown). Long-term-treated lepromatous patients showed a low level of the antibody to the lysate antigens (OD < 0.6), and paucibacillary patients and normal healthy individuals also had very little of the antibody (OD ~ 0.5).
The lysates produced from mouse macrophages on incubation with M. avium, M. intracellular, M. scrofulaceum, M. smegmatis, M. vaccae, M. kansasii, and M. tuberculosis do not preferentially bind with B + LL sera as compared to normal sera. With most of these lysates, there was slightly less binding to B + LL sera than to normal sera, indicating very little overlapping antigenic specificity in the antigens in these lysates compared to the antigens released in the presence of M. leprae. In spite of the higher binding ability of lysate with M. tuberculosis to B + LL sera, it could not discriminate normal sera from B + LL sera. The higher binding ability to both types of sera may be due to some other antigens not related to those of M. leprae. The positive reaction seen with M. tuberculosis lysate and B + LL sera may also be due to a different protein, as seen by the kaolin agglutination test. In this test, even after removal of antibodies with affinity to the antigens produced by M. tuberculosis by adsorption, the sera samples showed considerable affinity to the M. leprae-induced lysate but not to the M. tuberculosis-induced lysate.
The presence of live M. leprae leads to the introduction of some M. leprae products inside the host cells. These products could be identified using antibodies from the sera of leprosy patients. This enabled us to show the appearance of these antigen(s) of M. leprae inside a host cell as early as 24 hours after the start of incubation. This activity required live M. leprae, as indicated by our observations.
On treating the M. leprae-infected macrophages with cycloheximide, the lysate prepared still showed ELISA positivity. This suggested that the protein antigen is either produced by M. leprae or that it is a small peptide produced by the host macrophage which is not inhibited by cycloheximide. Since freezing and thawing could release preexisting M. leprae antigens, the experiment was performed with heat-killed and irradiated M. leprae incubated with macrophages. Under these conditions, the macrophage lysates showed no antigenic affinity to the B + LL sera. This indicated that pre-existing antigens were not simply being released by the process of freeze-thawing. The de novo synthesis of the antigen(s) by M. leprae was also indicated by the fact that rifampin could block the production of the antigcn(s). This is the first time that an M. leprae-related antigen, which seems to be a metabolic product of the bacillus, has been analyzed without extracting it from the bacteria. This opens up a variety of experimental possibilities, since the appearance of the anti-gen(s) may be related to the viability of M. leprae inside the host cells.
In another closely related paper (l0), we have shown that the antigen production was associated with viable M. leprae in the macrophages of Swiss white mice. On the other hand, in cultures of M. leprae-infected macrophages from C57BL mice, the viability of the M. leprae was reduced, and there was no production of the antigen. The viability of M. leprae in M. leprae-infected macrophage cultures appears to be genetically controlled in mice. Lysates from cultured human-blood macrophages infected with live M. leprae have been studied. If the macrophages were derived from the blood of long-term-treated LL patients, the lysates contain an antigen reacting with the B + LL serum. This is being studied further.
Acknowledgments. The authors wish to acknowledge the help from the Acworth Leprosy Hospital, Bombay, for serum samples and Dr. (Mrs.) U. Joshi, Institute of Research in Reproduction, Bombay, for use of the ELISA reader. The authors also wish to acknowledge financial grant D71/85 from the Department of Science and Technology, Government of India, for the support of the work reported here.
REFERENCES
1. BlRDI, T. J., MlSTRY, N. F., Mahadevan, P. R. and Antia, N. H. Alterations in the membrane of macrophages from leprosy patients. Infect. Immun. 41(1983)121-127.
2. Cho, S.-N., Yanagihara, D. L., Hunter, S. W., Gelber, R. H. and Brennan, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
3. Drutz, D. J. and Cline, M. J. Incorporation of 3H-thymidine by leprosy bacilli in cultures of human lepromatous macrophages. J. Infect. Dis. 125(1972)416-419.
4. Kurup, I. and Mahadevan, P. R. Cholesterol metabolism of macrophages in relation to the presence of Mycobacterium leprae. J. Biosci. 4(1982)307-316.
5. Lad, S. J. and Mahadevan, P. R. Adherence of Mycobacterium leprae to macrophage as an indicator of pathogen-induced membrane changes. Indian J. Med. Res. 76(1982)804-813.
6. Lad, S. J., Salgame, P. R. and Mahadevan, P. R. Surface membrane changes in lepromatous macrophages affecting the adherence of Mycobacterium leprae. J. Biosci. 5(1983)131-138.
7. Lowry, O. H., Rosebrough, N. J., Fair, A. L. and Randall, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
8. Marolia, J. and Mahadevan, P. R. Hydrolytic enzymes in macrophages from leprosy patients in presence of M. leprae. Indian J. Lepr. 56(1984)776-783.
9. Marolia, J. and Mahadevan, P. R. Reactive oxygen intermediates inactive Mycobacterium lep-rae in the phagocytes from human peripheral blood. Int. J. Lepr. 57(1989)483-491.
10. Nair, I., Varadkar, D. and Mahadevan, P. R. Viability of Mycobacterium leprae inside macrophages from different strains of mice and possible genetic control. Int. J. Lepr. 58(1990)548-553.
11. Prasad, H. K. and Nath, I. Incorporation of 3H-thymidine in Mycobacterium leprae with differentiated human macrophages. J. Med. Microbiol. 14(1981)279-293.
12. Sálgame, P. R., Birdi, T. J., Mahadevan, P. R. and Antia, N. H. Role of macrophages in defective cell-mediated immunity in lepromatous leprosy. I. Factor(s) from macrophages affecting protein synthesis and lymphocyte transformation. Int. J. Lepr. 48(1980)172-177.
13. Takahashi, Y. Specific scrum agglutination of kaolin particles sensitized with tubercle phosphatide and its clinical evaluation as a sérodiagnostic test for tuberculosis. Am. Rev. Respir. Dis. 85(1962)708-719.
14. Vejare, S. and Mahadevan, P. R. Importance of determining viability of Mycobacterium leprae inside macrophages-an in vitro method using uracil. J. Biosci. 11(1987)455-163.
15. Wheeler, P. R. Metabolism in Mycobacterium leprae; its relation to other research on M. leprae and to aspects of metabolism in other mycobacteria and intracellular parasites. Int. J. Lepr. 52(1984)208-230.
16. Young, D. B. A serological test for leprosy with a glycolipid specific for M. leprae. Science 221(1983)1057-1059.
1. M.Sc, The Foundation for Medical Research, 84-A R. G. Thadani Marg, Worli, Bombay 400018, India.
2. M.Sc, Ph.D., The Foundation for Medical Research, 84-A R. G. Thadani Marg, Worli, Bombay 400018, India.
Reprint requests to Dr. Mahadevan.
Received for publication on 23 Jun.e 1989.
Accepted for publication in revised form on 14 February 1990.