- Volume 58 , Number 3
- Page: 548–53
Viability of Mycobacterium leprae inside macrophages f rom different strains of mice and possible genetic control
ABSTRACT
Peritoneal macrophages f rom Swiss white mice in vitro tolerated Mycobacterium leprae and allowed metabolism of the bacteria leading to release of bacteria-specific antigenic protein. This was associated with the maintenance of viability of the bacilli inside the cells. Macrophages f rom C57BL mice reduced viability of M. leprae after phagocytosis, and this was associated with the production of superoxide. Blockage of superoxide production resulted in maintaining viability of the cells of these mouse strains. Associated with loss of viability of the bacilli is the absence of the production of antigenic protein in the lysate. Interestingly, the maintenance of viability or loss of viability and the factors controlling such viability in the macrophages of Swiss white and C57BL mice, respectively, appeared to be genetically controlled.RÉSUMÉ
Des macrophages péritonéaux de souris blanches Swiss ont toléré Mycobacterium leprae in vitro, et permis le métabolisme des bactéries conduisant à la libération de protéines antigéniques spécifiques à la bactérie. Ceci était associé au maintien de la viabilité des bacilles à l'intérieur des cellules. Les macrophages des souris C57BL ont diminué la viabilité de M. leprae après phagocytose, et ceci était associé à la production de superoxyde. Le blocage de la production de superoxyde a résulté dans le maintien de la viabilité des cellules de ces souches de souris. L'absence de production de protéine antigénique dans le lysat est associée à la perte de viabilité des bacilles. Il est intéressant de noter que le maintien de la viabilité ou la perte de viabilité et les facteurs contrôlant cette viabilité dans les macrophages des souris blanches SWISS et C57BL respectivement, sont apparus être sous contrôle génétique.RESUMEN
Los macrófagos peritoncales de ratones Swiss-white toleraron in vitro el crecimiento de Mycobacterium leprae y permitieron su metabolismo que condujo a la liberación de proteínas antigénicas específicas de la bacteria. Esto estuvo asociado con el mantenimiento de la viabilidad de los bacilos dentro de las células. Los macrófagos de los ratones C57BL redujeron la viabilidad del M. leprae después de la fagocitosis, y esto estuvo asociado con la producción de superóxido. El bloqueo de la producción de superóxido permitió el mantenimiento de la viabilidad bacteriana en las células de esta cepa de ratón. La pérdida de viabilidad de los bacilos estuvo asociada a la pérdida de la producción de proteínas antigénicas en los lisados. Interesantemente, los factores que controlan el mantenimiento o la pérdida de la viabilidad de M. leprae en los macrófagos de los ratones Swiss-white y C57BL, respectivamente, parecen estar genéticamente controlados.Murine models for the study of Mycobacterium leprae have been adopted during the last three decades (8). Associated with this is the observation that all strains of mice do not support the growth of M. leprae-murium in a uniform manner (3). Among those mouse strains which allow a discernible level of M. leprae growth in the foot pad are the Swiss white (SW), BALB/c, and CBA strains. Among the mouse strains that allow very restricted growth is the C57BL strain. Comparatively higher resistance to M. leprae in C57BL mice has been reported earlier (2,13). C57BL strains of mice are also reported to be resistant to M. lepraemuriuin as compared to the C3H strain (3,5). In some mouse strains, resistance to salmonella, leishmania, and BCG is recorded as due to genetic control mediated through a gene on chromosome 1 (15). Except for a general idea of different behavior of mouse strains to M. leprae growth, there is no substantial evidence to indicate resistance or susceptibility of different strains of mice to M. leprae infection.
In this paper, we present data to show that the peritoneal macrophages of two different strains of mice deal with phagocy-tosed M. leprae differently. One is able to kill the bacilli; the other is unable to do so. Such a phenomenon appears to be genetically controlled, indicating an inherent susceptibility in one mouse strain -the SW type. Further, we also provide data which demonstrate that the viability of M. leprae in these mice is related to the failure of their macrophages to produce reactive oxygen intermediates, especially superoxide. The viability of M. leprae inside the cultured peritoneal macrophages was indicated by production of some bacterial-specific antigens with binding ability to scrum antibodies from bacteriologically positive leprosy patients (11). That this is a specific metabolic activity of M. leprae inside the phagocyte is also reported in our companion paper (11). In this paper, we tested the ability of M. leprae to produce antigenic proteins in cultures of macrophages from two mouse strains and their F1 and F2 progeny obtained after a genetic cross. These experiments were to ascertain if there was a genetic control in mice in relation to resistance to M. leprae inside the mononuclear phagocytes.
MATERIALS AND METHODS
Mice. In-house, randomly bred, Swiss white mice (Rockefeller) (SWR) and inbred C57BL/6 mice (Jackson strain) were used. Genetic studies using breeding pairs between these two strains and their F1 and F2 progeny were carried out. Both strains were used as female parents in the breeding pairs.
Preparation of macrophage cultures. For all of the experiments, resident peritoneal macrophages from the above mouse strains and their progeny were used. The mice were sacrificed by cervical dislocation and 5 ml of Minimal Eagle's Medium (MEM) with 20% inactived human serum was injected into the peritoneal cavity. The surface of the abdomen was agitated, and the peritoneal fluid was withdrawn. The cell suspension was distributed in petri plates (Becton Dickinson 3002) in volumes that would give approximately 1 x 106 cells/petri plate as judged by our previous experience. After 24 hr the plates were washed and infected with 30 x 106 M. leprae for 1 x 106 macrophages. After a 24-hr incubation, excess M. leprae were washed off, and the cells scraped and lysed by 10 cycles of freeze-thawing, taking care to see that the macrophages were lysed, releasing the lysate without damaging the M. leprae. M. leprae were obtained from infected armadillo tissues. The collection, storage of the tissues, and preparation of the M. leprae have been reported (11).
Preparation of lysates. Lysed macrophages were centrifuged at 21,000 x g x 30 min in a Sorvall RC5B refrigerated su-perspeed centrifuge. The supernatant was passed through a Millipore filter (0.22 μm) to assure complete removal of M. leprae and to obtain sterile samples. Protein was estimated by the method of Lowry, et al. (7).
Viability of M. leprae. The viability of M. leprae inside the macrophages after phagocytosis was determined by the fluorescein diacetate (FDA; Sigma Chemical Co., U.K.) staining method described earlier (18) which was a modification of that of Kvach, et al. (4). The cells infected with M. leprae were scraped off the culture dish and lysed by 10 cycles of freeze-thawing to release the bacilli. A suspension of M. leprae (0.003 ml) was then placed on a slide and incubated in the dark with 4 μg/ml of FDA and 4 μg/ml of ethidium bromide (Sigma, U.K.) for 30 min. The suspension was then examined by a fluorescence microscope under UV light (Fluoval II). A parallel aliquot from the same preparation was smeared on the slide and stained for acid-fast bacilli (AFB). The AFB count gave the total number of bacilli, and the green-fluorescing bacilli (GFB) indicated the proportion of viable bacilli in the total population. The quantum of viability was expressed as percent GFB in a total AFB population.
Mouse foot pad tests. The viability of M. leprae after phagocytosis was determined by recovering the bacilli from the cells and testing their ability to grow in the foot pads of SW mice. The recovery of the bacilli was done after freeze-thawing of the cells 10 times and centrifugation. A bacterial suspension of 1 x 104 bacilli was used for inoculation into the foot pad, and the growth obtained at 8 and 12 months postinoculation was determined. The method of counting the bacilli after foot-pad harvest was based on that of Shepard and McRae (14). The mouse foot pad test was done to correlate the loss or retention of viability in the same populations of bacteria that were also tested by the FDA method. We have not determined the most probable number of viable M. leprae inside the cells using the mouse foot pad method.
Superoxide anion estimation. To estimate the ability of the cells to produce superoxide anion (superoxide), peritoneal macrophage cultures were prepared as mentioned earlier. On the day of termination, the cultures, after repeated washing with MEM, were challenged with 25 x 106 live M. leprae. The superoxide released during 3 hr after exposure to M. leprae was measured using 0.3% nitroblue tetrazolium (Sigma, U.K.) based on the method of Sugi-moto, et al. (17) and as modified by Marolia and Mahadevan (9). The superoxide released was expressed as nmole/hr/106 cells in the culture in the presence or absence of superoxide dismutase (100 μg/petri dish; Sigma, U.K.).
Statistical significance was calculated using Student's t test. Average values are given along with standard deviations.
RESULTS
Production of M. leproe-specific proteins. As has been reported earlier, it could be demonstrated that macrophages from SW mice on incubation with M. leprae produced in the cell lysate proteins with binding ability to antibodies present in the sera of bacteriologically positive leprosy patients (responders). This was determined by an ELISA as optical density (OD 492 nm). The intensity of the OD indicated the concentration of the antibody to the particular protein under test on infection with viable M. leprae (Fig. 1). Sera from normal individuals had no antibodies with binding ability. However, the lysate produced on incubating M. leprae with macrophages from C57BL/6 mice did not show any antigens with binding ability to sera from leprosy patients (nonresponders). This was unlike the lysate obtained from macrophages of SW mice. Our preliminary observation by Western blot showed a faint spot reacting to serum from a lepromatous leprosy patient (data not shown). Significantly, this failure to produce the antigcn(s) by C57BL/6 macrophages was genetically controlled. All F1 generation mice (20) behaved as C57BL/6 mice, and in the F2 generation, the character seems to segregate in a classical 3:1 ratio, 11 being producers and 4 nonproducers of antigen in M. leprae-infected macrophage cultures in a total of 15 animals (p < 0.0001) (Fig. 1).
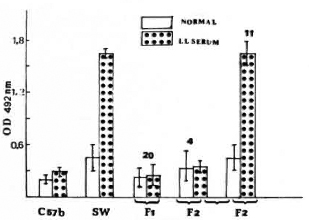
Fig. 1. Differential ability of M. leprae-infected, mouse peritoneal macrophages to produce antigenic protein with binding ability to bacteriologically positive lepromatous leprosy patients' sera and no binding ability to normal sera. The macrophages were obtained from parents and progeny of a cross between C57BL and Swiss white mice.
M. leprae viability. The viability of M. leprae, as determined by FDA staining, inside the macrophages obtained from the two strains of mice used as parents and the F1 and F2 progeny mice is presented in Figure 2. In macrophages from SW mice, M. leprae with an original viability of approximately 40% were used and, after phagocytosis, they had an average viability of 30%. This indicated the resistance of viable M. leprae and the inability of the cells from SW mice to reduce the viability. However, in the macrophages from C57BL mice, the original viability of the bacilli of approximately 40% was reduced to 7%. All of the 20 F1 mice showed a M. leprae viability pattern similar to the parent C57BL strain. The F2 progeny showed a segregation with four mice showing reduction in viability as in the C57BL macrophages and 11 showing inability to reduce M. leprae viability as in the macrophages from SW mice.
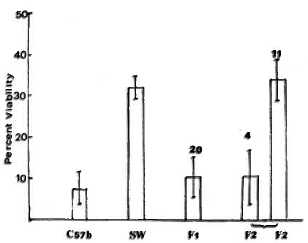
Fig. 2. Differential viability of phagocytosed A/. leprae as determined by fluorescein diacetate (FDA) test inside macrophages from different mouse strains (as parents) and mice obtained in Fl and F2 generation in the cross between C57BL and Swiss white strains. Average smear viability of M. leprae used for phagocytosis was 35%-40% as determined by FDA test.
Mouse foot pad growth. To correlate the loss of viability as indicated by the FDA test, M. leprae phagocytosed and incubated inside the macrophages of both types of mice were recovered, and on testing their ability to grow in the mouse foot pad, the results showed that only M. leprae derived from SW macrophages had viable bacilli as indicated by growth at the 8, 10, and 12 months' harvests. In this case there was a slow but definite increase in growth from the M. leprae obtained from these macrophages. However, no growth occurred in the foot pad when bacteria phagocytosed by macrophages from C57BL mice were used, indicating a loss of viable bacilli incubated in the macrophages from this group of mice (Table 1).
Superoxide production and viability. Cultured peritoneal macrophages on challenge with live M. leprae produce reactive oxygen metabolites like superoxide. This is clearly indicated in the data presented in Table 2. The level of superoxide produced by macrophages from C57BL mice is significantly higher than that released from the cells of SW mice.
Associated with this production of superoxide is reduced viability of M. leprae in macrophages from C57BL mice but not in macrophages from SW mice. Superoxide dismutase removes all of the superoxide produced on challenge with M. leprae, and the loss of viability seen in macrophages from C57BL mice was prevented in the presence of superoxide dismutase (Table 2).
Macrophages from the parent SW mice showed a poor ability to produce superoxide as compared to macrophages from the other parent, C57BL/6 mice. Macrophages from all of the F1 progeny behaved like macrophages from the C57BL/6 parents with release of a higher amount of superoxide, but F2 animals showed segregation of the character. In the F2 generation only five animals were tested.
DISCUSSION
We have reported that in vitro cultured peritoneal macrophages of SW mice infected with live M. leprae are able to produce some antigenic proteins with binding affinity to antibodies in the sera of lepromatous patients (11). For the production of such proteins, one needed live M. leprae. In this paper, we have extended these observations and shown that macrophages from comparatively resistant C57BL mice, after phagocytosis of M. leprae, were able to kill the bacilli in association with the production of superoxide. Superoxide seems to play an important role in the loss of viability, since removal of the superoxide with superoxide dismutase prevented the loss of viability. Earlier, Marolia and Mahadevan (10) showed the importance of superoxide and hydroxyl radical ions in the killing of M. leprae inside phagocytes of normal healthy individuals, and that due to the lack of superoxide production, the viability of M. leprae was maintained in the cells of leprosy patients. A parallel situation was observed with macrophages from C57BL and SW mice. Due to this loss of viability of the bacteria, the cells do not have any M. leprae protcin(s) showing binding affinity to the antibodies of leprosy patients. In the macrophages of SW mice viability is maintained, associated with the lack of superoxide production. This led to the production of the antigenic protein reported earlier (11) and also in the present paper.
Interestingly, this tolerance of M. leprae by SW macrophages and the loss of M. leprae viability in the macrophages of C57BL mice and the associated features appeared to be under genetic control. All F1 animals in a cross between the SW and C57BL mouse strains behaved like the parent C57BL mice regarding all three features, i.e., ability of cultured macrophages to produce superoxide on in vitro challenge with M. leprae, loss of viability of M. leprae in M. leprae-infected macrophages in vitro, and absence of M. leprae-induced antigenic protein(s) in Iysates of infected macrophages. In the F2 progeny, all of these three characters were shown similar to that of the C57BL mice, but only in 3 out of 4 mice, and in one the features were similar to that of the parent SW mice. Thus, macrophage function as measured by these three parameters appeared to be under genetic control. The genetic influence may be correlated to the susceptibility to M. leprae in SW mice and to the resistance to M. leprae in the cells of C57BL mice.
The natural resistance of mice to infection with Salmonella typhimurium and Leish-mania donovani is controlled by chromosome 1 gene(s) designated Ity and Lsh, respectively (12,15). These two pathogenic organisms were taxonomically and antigen-ically distinct, and yet the host response to them appears to be regulated by the same locus or complex. Therefore, one might expect that the resistance to other intracellular pathogens could be controlled similarly through a common mechanism. Innate resistance of inbred mice to infection with BCG is regulated by the BCG gene, and the distribution of the mouse strains matched exactly that established for resistant and susceptible alleles of Lsh and Ity, suggesting coordinated control by the same locus of chromosome 1 (16).
However, unlike BCG susceptibility in the C57BL/6 strain with no indication of resistance, we found that our C57BL/6 strain shows a good degree of resistance to M. leprae. Earlier, poorer growth of M. leprae in the foot pads of C57BL/6 mice had been reported (2,13). Our observations with M. leprae appear to parallel this genetic control of susceptibility in mice reported for M. lep-raemurium to the extent that the C57BL mouse strain is also more resistant to M. leprae (3,6). Our results do indicate the role of macrophages in relation to M. leprae viability and its relation to reactive oxygen intermediates like superoxide. These observations are very complementary to what has been reported in relation to human patients (9). Accordingly, further studies on the mechanism by which the gene exerts its effects in relation to M. leprae infection could reasonably be expected to shed considerable light on how macrophages help to control infections in leprosy.
Acknowledgment. The authors wish to acknowledge financial grant D71/85 from the Department of Science and Technology, Government of India, for the support of the work published here.
REFERENCES
1. Bhagaria, A. and Mahadevan, P. R. A rapid method for viability and drug sensitivity of M. leprae cultured in macrophages and using fluores-cein-diacetate. Indian J. Lepr. 59(1987)9-19.
2. Birdi, T. J., Sálgame, P. R. and Antia, N. H. The role of macrophages in leprosy as studied by protein synthesis of macrophages from resistant and susceptible hosts-a mouse and human study. Lepr. India 52(1979)23-42.
3. Closs, O. Experimental murine leprosy: growth of Mycobacterium lepraemurium in C3H and C57BL mice after foot pad inoculation. Infect. Immun. 12(1975)480-489.
4. Kvach, J. T., Munguia, G. and Strand, S. H. Staining tissue-derived Mycobacterium leprae with fluorescein diacetate and ethidium bromide. Int. J. Lepr. 52(1984)176-182.
5. Lagrange, P. H. and Hurtrel, B. Local immune response to Mycobacterium lepraemurium in C3H and C57BL/6 mice. Clin. Exp. Immunol. 38(1979)461-474.
6. Lovik, M. Experimental murine leprosy and its relevance for the study of resistance to mycobacterial infections in man. Int. J. Lepr. 55(1987)689-701.
7. Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
8. Mahadevan, P. R., Jagannathan, R., Bhagaria, A., Vejare, S. and Agarwal, S. Host-pathogen interaction -new in vitro drug test systems against Mycobacterium leprae; possibilities and limitations. Lepr. Rev. 57Suppl.3(1986)182-200.
9. Marolia, J. and Mahadevan, P. R. Superoxide production from macrophages of leprosy patients after stimulation with Mycobacterium leprae. J. Biol. Sci. 12(1987)273-279.
10. Marolia, J. and Mahadevan, P. R. Reactive oxygen intermediates inactivate Mycobacterium leprae in the phagocytes from human peripheral blood. Int. J. Lepr. 57(1989)483-91.
11. Nair, I. and Mahadevan, P. R. Antigenic protein from Mycobacterium leprae released in macrophages in vitro as indicator of viability of bacteria. Int. J. Lepr. 58(1990)540-547.
12. Plant, J. E., Blackwell, J. M., O'Brien, A. D., Bradley, D. J. and Glynn, A. A. Are the Lsh and Ity disease resistance genes at one locus on chromosome 1? Nature 297(1982)510-513.
13. Shepard, C. C. and Habas, J. A. Relation of infection to tissue temperature in mice infected with M. marinum and M. leprae. J. Bacteriol. 93(1967)790-796.
14. Shepard, C. C. and McRae, D. H. Mycobacterium leprae in mice: minimum infectious dose, relationship between staining quality and infectivity and effect of cortisone. J. Bacteriol. 89(1965)365-372.
15. Skamene, E., Gros, P., Forget, A., Kongshavn, P. A. L., St. Charles, C. and Taylor, B. A. Genetic regulation of resistance to intracellular pathogens. Nature 297(1982)506-509.
16. Skamene, E., James, S. L., Meltzer, H. S. and Nesbitt, M. N. Genetic control of macrophage activation for killing of extracellular targets. J. Leukocyte Biol. 35(1984)65-69.
17. Sugimoto, M., Higuchi, S., Ando, M., Horio, S. and Tokuomi, H. The effect of cytochalasin B on the superoxide production by alveolar macrophages obtained from normal rabbit lungs. J. Reticuloendothel. Soc. 31(1982)117-130.
1. M.Sc; The Foundation for Medical Research, 84-A R.G. Thadani Marg, Worli, Bombay 400018, India.
2. M.Sc.; The Foundation for Medical Research, 84-A R.G. Thadani Marg, Worli, Bombay 400018, India.
3. M.Sc, Ph.D., The Foundation for Medical Research, 84-A R.G. Thadani Marg, Worli, Bombay 400018, India.
Reprint requests to Dr. Mahadevan.
Received for publication on 23 June 1989.
Accepted for publication in revised form on 14 February 1990.