- Volume 58 , Number 3
- Page: 554–9
Ocular leprosy in nine-banded armadillos following intrastromal inoculation
ABSTRACT
Leprosy shows a higher percentage of ocular involvement than any other systemic infection. In humans, the cornea is the first ocular tissue affected. Our previous studies in armadillos with naturally acquired and experimental disseminated leprosy showed that 44% had corneal infection. Mycobacterium leprae is found in armadillo burrows in Louisiana, U.S.A., and ocular abrasions may be the portal of entry for these organisms in wild armadillos. To test the cornea as a route of infection, we injected eight armadillos intrastromally with 2 x 106 M. leprae in 1 μl. Two and 4 months later, the armadillos were sacrificed and their eyes processed for light- and electron-microscopy. After 2 months, M. leprae were found in histiocytes mainly in the corneal limbus, sclera and bulbar conjunctiva. At 4 months, however, there was a visible corneal leproma in one animal. Microscopically, it was found to be a histiocytic granuloma with heavy M. leprae invasion. In addition, cells were seen in the anterior chamber. Leprosy is endemic in regions where other corneal infections which compromise the epithelial barrier property are prevalent and where leprosy bacilli are found in the environment. The entry of leprosy bacilli into the cornea may produce lesions which spread posteriorly in the eye.RÉSUMÉ
La lèpre montre un pourcentage d'atteinte oculaire plus élevé qu'aucune autre infection systémique. Chez l'homme, la cornée est le premier tissu oculaire atteint. Nos études antérieures chez des tatous ayant une lèpre disséminée naturelle ou expérimentale ont montré que 44% avaient une infection oculaire. On trouve Mycobacterium leprae dans des terriers de tatous en Louisiane, U.S.A., et des abrasions oculaires pourraient être la porte d'entrée de ces organismes chez les armadillos sauvages. De manière à tester la cornée comme porte d'entrée à l'infection, nous avons injecté 1 μl contenant 2 x 106 M. leprae dans le stroma de huit tatous. Les tatous furent tués deux et quatre mois plus tard, et leurs yeux préparés pour examen au microscope oculaire et électronique. Après deux mois, M. leprae fut trouvé dans les histiocytes, principalement dans le limbe corneal, la sclérotique et la conjonctive bulbaire. A 4 mois, cependant, il y avait un léprome cornéen visible chez un animal. Au microscope, on observa qu'il s'agissait d'un granulome histiocytaire fortement envahi par M. leprae. De plus, des cellules furent observées dans la chambre antérieure. La lèpre est endémique dans des régions où d'autres infections cornéennes qui compromettent la barrière épithéliale sont prévalentes, et où des bacilles de lèpre sont trouvés dans l'environnement. L'entrée des bacilles de lèpre dans la cornée pourrait produire des lésions qui se disséminent plus en arrière dans l'oeil.RESUMEN
La lepra muestra el mayor porcentaje de afección ocular que cualquier otra infección sistémica. En humanos, la córnea es el primer tejido ocular afectado. Nuestros estudios previos en armadillos con lepra diseminada adquirida en forma natural o experimental, mostraron un 44% de afección corneal. Mycobacterium leprae se encuentra en las madrigueras de los armadillos en Louisiana, U.S.A. y las abrasiones oculares pueden ser la puerta de entrada de los organismos en los armadillos salvajes. Para probar la córnea como una ruta de infección, inyectamos 8 armadillos intraes-tromalmente con 2 x 106 M. leprae en 1 μl. Dos y 4 meses después, los armadillos se sacrificaron y sus ojos se prepararon y procesaron para microscopía de luz y electrónica. Después de 2 meses, se encontraron M. leprae en los histiocitos del limbus corneal, en la esclera y en la conjuntiva bulbar. A los 4 meses apareció un leproma corneal visible en un animal. Microscópicamente éste resultó ser un granuloma histiocítico con invasión masiva por M. leprae. También se llegaron a observar células en la cámara anterior. La lepra es endémica en regiones donde prevalecen otras infecciones corneales que comprometen la integridad de la barrera epitelial y donde el bacilo de la lepra se encuentra en el ambiente. La entrada del bacilo de la lepra en la córnea puede producir lesiones que después se dispersan en el ojo.Leprosy shows a higher percentage of ocular involvement than any other systemic infection. The ocular complications of the disease arc manifest in as many as 90% of leprosy patients, and they frequently lead to blindness (4-6). By World Health Organization standards, leprosy is a cause of blindness in nearly one million patients (15), however, blindness prevention programs have paid little attention to the prevention of this disabling complication. The ocular manifestations are mainly in the anterior segment and vary with the different clinical types of leprosy. In tuberculoid leprosy, there is mainly 5th and 7th cranial nerve involvement resulting in corneal anesthesia and lag-ophthalmos, while in the lepromatous end of the spectrum there is direct bacillary invasion of the cornea and anterior uvea with interstitial keratitis and iritis. The pathogenetic mechanisms that result in these lesions are not clearly defined. Studies in animal models have revealed histopathological changes similar to those described in human leprosy (2,7,9,14). In a study on mangabey monkeys with lepromatous leprosy, the histopathological changes in the eye were those previously described in the early stages of the disease in humans (2,12). Corneal manifestations, such as the thickening and beading of corneal nerves, are early manifestations of the disease, and may be the presenting clinical signs of leprosy (1).
The mode of transmission of leprosy is not yet determined and the cornea is a possible portal of entry for organisms. Leprosy is endemic in geographical areas where corneal diseases which compromise the epithelial barrier properties are prevalent (15). Also, Mycobacterium leprae has been isolated from the environment (3).
The objective of this study was to test the hypothesis that a compromised corneal epithelial barrier could be a putative route of entry of M. leprae. To this end, we injected M. leprae into the corneal stroma of nine-banded armadillos and observed the development of ocular pathology at intervals following inoculation.
MATERIALS AND METHODS
Eight nine-banded armadillos that were laboratory adapted were used in this study. They were screened and found negative for infection with M. leprae by nasal smears and skin smears stained for acid-fast bacilli (AFB).
The inoculum was a partially purified suspension of M. leprae obtained from the foot pads of athymic nude mice. This was kindly provided by Dr. Scott Franzblau (Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A.). The bacillary count was 2 x 109 per ml.
Prior to corneal inoculation, the animals were anesthetized by intramuscular injection of 2 ml ketamine hydrochloride (80 mg/ml). One microliter was injected intra-stromally, using a Hamilton syringe and a ¼ inch 33-gauge needle. One eye was injected with the M. leprae suspension (approximately 2 x 106 organisms) and the contralateral eye with the same volume of phosphate buffered saline (PBS) as control. The eyes were examined after 3 days and monthly thereafter.
Four animals were sacrificed by an intravenous overdose of pentobarbital 2 months after inoculation; the other four, after 4 months. The eyes were enucleated, fixed in 1% paraformaldehyde 2.5% glutaraldehyde 0.01 % picric acid in a 0.1 M cacodylate buffer, pH 7.4, and processed for light- and electron-microscopy. All animals were examined for systemic M. leprae infection by nasal and skin smears.
Tissues were dehydrated in graded series of ethanol and embedded in glycolmethac-rylate (Historesin; LKB-Bromma, Sweden) for light-microscopy. Two micron sections were stained with a modified acid-fast stain (13). This modification was previously developed and used by one of the authors (RM) in conjunction with plastic-embedded tissue. Briefly, staining was preceded by treatment with periodic acid for 30 min, then staining with carbol fuchsin for 8 min, de-colorization with 1% sulfuric acid in 70% ethyl alcohol, and a counterstain with methylene blue 0.14% for 2 min. For electron-microscopy, tissues were postfixed in 1% osmium tetroxide, dehydrated, and embedded in Epon. Thin sections were cut on a Sorvall MT2-B ultramicrotome, stained with uranyl acetate and lead acetate, and viewed in a Zeiss EM 10C/A transmission electron microscope.
RESULTS
The injection procedure produced a restricted stromal opacity which resolved within 3 days. Macroscopic examination of the eyes 2 months postinoculation revealed no relevant abnormalities. Microscopic examination, however, revealed a mononuclear cell infiltrate at the corneal limbus, in the bulbar conjunctiva, sclera, and at the angle of the anterior chamber (Fig. 1). The modified acid-fast stain revealed AFB, mostly solid stained, in histiocytes in the stroma of the limbus and the sclera (Fig. 2). Some of the corneal nerves were surrounded by an infiltrate of histiocytes and lymphocytes. There were also perivascular inflammatory cells around the limbal vessels; however, no AFB were found at these sites.

Fig. 1. Corneal limbus of an armadillo 2 months following intrastromal inoculation with M. leprae. Note the mononuclear cell infiltrate just below the bulbar conjunctiva (arrows) (modified acid-fast stain x 125).
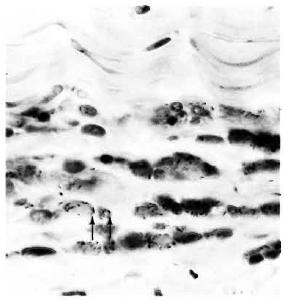
Fig. 2. Acid-fast bacilli in the stroma of corneal limbus (arrows) of an armadillo inoculated intrastromally with M. leprae 2 months previously (modified acid-fast stain x 950V
After 4 months, macroscopic examination revealed corneal opacity in three of the animals. Another animal showed a frank leproma similar to those observed in human lepromatous leprosy cases (Fig. 3). Light-microscopy revealed an extensive histiocytic infiltrate in the corneal stroma. This infiltrate extended to the iris and ciliary body, which appeared swollen and necrotic (Fig. 4) with some cells detached in the anterior chamber and on the corneal endothelium. Many of the histiocytes contained clumps of AFB (Fig. 5). The corneal nerves were surrounded by an inflammatory infiltrate, but no AFB were found. By electron-microscopy, leprosy bacilli were observed in keratocytes and macrophages in the corneal stroma (Fig. 6). The bacilli were surrounded by an electron transparent zone that is characteristic for M. leprae. Leprosy bacilli were also found in macrophages and pigmented cells of the iris and ciliary body.
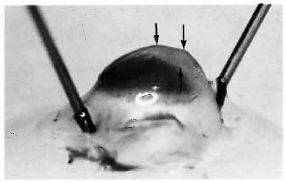
Fig. 3. Eye of armadillo at necropsy 4 months after intrastromal inoculation of M. leprae. Note corneal leproma (arrows).
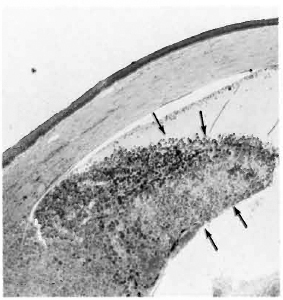
Fig. 4. Anterior ocular segment of an armadillo injected 4 months previously with M. leprae in the corneal stroma. The iris and ciliary body are markedly swollen and necrotic (modified acid-fast stain x50).
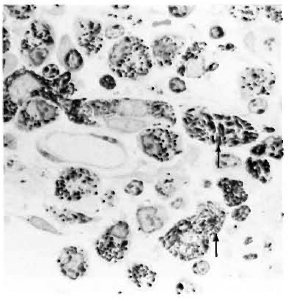
Fig. 5. Acid-fast bacilli (arrows) in histiocytes in the ciliary body of an armadillo injected intrastromally with M. leprae 4 months previously (modified acid-fast stain x 1500).
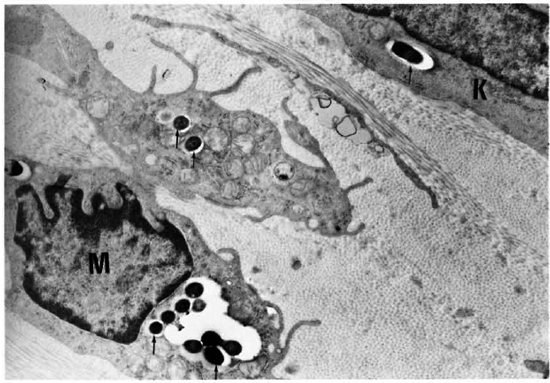
Fig. 6. Electron micrograph of the cornea of an armadillo injected intrastromally with M. leprae 4 months previously. Note the macrophage (M) and keratocytc (K) with leprosy bacilli surrounded by the electron transparent zone (arrows) ( x3200).
DISCUSSION
In this study, we established a M. leprae infection in the cornea and anterior uvea of armadillos following intrastromal inoculation. Two months' postinoculation, leprosy bacilli were already localized in the limbus and sclera and were found in areas of chronic inflammatory cellular infiltrates. The bacilli at that stage were found mainly in macrophages. After 4 months, however, the macroscopic and microscopic changes were more evident. A corneal leproma similar to those observed in patients with leproma-tous leprosy (15) was observed in one animal. Microscopically, the extensive corneal and uveal inflammatory infiltrate as well as the invasion by M. leprae is comparable to the microscopic findings seen in patients with advanced lepromatous leprosy (11) and in armadillos with systemic infection. (13,14) In these previous studies, M. leprae were observed in the corneal stroma localized in keratocytes, endothelial cells of blood vessels, and in Schwann cells surrounding myelinated and unmyelinated corneal nerves (13). Sixty percent of systemically infected animals show uveal tract involvement with M. leprae in the ciliary epithelium and ciliary muscle and in blood vessels in the iris (14).
Corneal manifestations are early signs of the ocular complications of leprosy (1) and are sometimes the presenting clinical feature of the disease. The mode of access for M. leprae to ocular tissues, however, is not known. A compromised cornea is a possible route of entry of organisms to the eye, especially since leprosy coexists with other ocular infections and nutritional deficiencies that compromise the corneal epithelial barrier properties. In leprosy-endemic areas, the etiological agent has been isolated from the environment (3). Armadillo burrows in Louisiana may also be a source of infection, as evidenced by the detection of M. leprae-specific antigen in soil samples from these burrows (16). Another source of evidence for transmission of M. leprae from the environment was the detection of lepromatous granulomas around thorns in the ears and noses of wild armadillos, suggesting that thorn pricks were a means of transmission of leprosy (8). Similarly, these environmental factors may be a source of infection and may aid in the access of M. leprae to the ocular tissues of wild armadillos.
The rapid development of corneal lesions following intrastromal inoculation is remarkable since a subcutaneous or intradermal injection of a M. leprae suspension in armadillos generally does not yield lesions before 6 months (17).
The mode of extension of M. leprae infection from the stroma to intraocular tissues cannot be clearly defined at this stage of our experiments. The hematogenous or the neural route are possible since both perivascular and perineural infiltrates were observed; however, an examination of the tissues at earlier postinoculation periods would help to clarify this. Our previous studies have revealed neural and vascular involvement in the corneas of systemically infected armadillos; however, these animals already had an advanced systemic infection (13). In light of the above-mentioned findings regarding environmental sources of M. leprae, and the compromise of the cornea in leprosy-endemic areas, it is possible that M. leprae may gain access to ocular tissues via the cornea.
Acknowledgment. This research was supported in part by National Institutes of Health grants EY07213, EY04074, and EY02377, and the National Eye Institute.
REFERENCES
1. Allen, J. H. and Byers, J. L. The pathology of ocular leprosy: I. Cornea. Arch. Ophthalmol. 64(1960)216-220.
2. Baskin, G. B., Wolf, R. H., Gormus, B. J., Meyers, W. M. and Malaty, R. Experimental leprosy in the mangabey, Cerocebus atys: necropsy findings. Int. J. Lepr. 53(1985)269-277.
3. Blake, L. A., West, B. C, Lary, C. H. and Todd, J. R., IV. Environmental nonhuman sources of leprosy. Rev. Infect. Dis. 9(1987)562-577.
4. Choyce, D. P. Discussion on ocular leprosy. Trans. R. Soc. Trop. Med. Hyg. 64(1970)43-45.
5. Courtright, P. A survey of the eye complications of leprosy in South Korea. Lepr. Rev. 55(1984)229-237.
6. Hobbs, H. E. and Choyce, D. P. The blinding lesions of leprosy. Lepr. Rev. 42(1971)131-137.
7. Hobbs, H. E., Harman, D. J„ Rees, R. J. W. and McDougall, A. C. Ocular histopathology in animals experimentally infected with Mycobacterium leprae and M. lepraemurium. 1. Mycobacterium leprae and M. lepraemurium infections in the mouse. 2. Mycobacterium leprae infections in the 9-banded armadillo (Dasypus novemcinclus L.). Br. J. Ophthalmol. 62(1978)516-524.
8. Job, C. K., Harris, E. B., Allen, J. L. and Hastings, R. C. Thorns in armadillo ears and noses and their role in the transmission of leprosy. Arch. Pathol. Lab. Med. 110(1986)1025-1028.
9. Kirchheimer, W. F. and Storrs, E. E. Attempts to establish the armadillo as a model for the study of leprosy. I. Report of lepramatoid leprosy in an experimentally infected armadillo. Int. J. Lepr. 39(1971)693-702.
10. Leininger, J. R., Donham, K. J. and Meyers, W. M. Leprosy in a chimpanzee: postmortem lesions. Int. J. Lepr. 48(1980)414-421.
11. Malaty, R., Hidayat, A. A. and Meyers, W. M. Ocular leprosy: a light and scanning eletron microscopic study (Abstract) Invest. Ophthalmol. Vis. Sci. 26Suppl.(1985)67.
12. Malaty, R., Meyers, W. M., Walsh, G. P., Binford, C. H., Zimmerman, L. E., Baskin, G. B., Gormus, B. J., Martin, L. N. and Wolf, R. H, Histopathological changes in the eyes of man-gabcy monkeys with lepromatous leprosy. Int. J. Lepr. 56(1988)443-448.
13. Malaty, R. and Togni, B. Corneal changes in nine-banded armadillos with leprosy. Invest. Ophthalmol. Vis. Sci. 29(1988)140-145.
14. Malaty, R., Walsh, G. P., Meyers, W. M., Binford, C. H. and Job, C. K. Ocular leprosy in nine banded armadillos. (Abstract) Invest. Ophthalmol. Vis. Sci. 27Suppl.(1986)38.
15. Schwab, I. R., Nassar, E., Malaty, R., Zarifa, A., Korra, A. and Dawson, R. Leprosy in a trachomatous population. Arch. Ophthalmol. 102(1984)240-244.
16. Truman, R. W., Franzblau, S. G. and Job, C. K. The nine-banded armadillo, Dasypus novem-cinctus, as an animal model to study the transmission of leprosy. (Abstract) In: Abstracts of the American Society for Microbiology 86th Annual Meeting. Washington, D.C.: American Society for Microbiology, 1986, p. 123.
17. Walsh, G. P., Meyers, W. M. and Binford, C. H. Leprosy as a zoonosis: an update. Acta. Leprol. (Genève) 6(1988)51-60.
1. M.D., Ph.D.; Lions Eye Research Laboratories, LSU Eye Center, Louisiana State University School of Medicine, New Orleans, Louisiana, U.S.A.
2. Ph.D.; Lions Eye Research Laboratories, LSU Eye Center, Louisiana State University School of Medicine, New Orleans, Louisiana, U.S.A.
3. Lions Eye Research Laboratories, LSU Eye Center, Louisiana State University School of Medicine, New Orleans, Louisiana, U.S.A.
Reprint requests to: Raga Malaty, M.D., Ph.D., LSU Eye Center, 2020 Gravier Street, Suite B, New Orleans, Louisiana 70112, U.S.A.
Received for publication on 5 January 1990.
Accepted for publication in revised form on 7 March 1990.