- Volume 58 , Number 3
- Page: 560–5
Major histocompatibility complex class ii antigen expression in nerves in leprosy; an immunoelectronmicroscopical study
ABSTRACT
A technique for immunoelectronmicros-copy has been used to investigate major histocompatibility class II expression in leprosy nerves. In normal nerves, endothelial cells and occasional endoneural cells (not Schwann cells) were constitutively class II positive. In both paucibacillary and multibacillary leprosy nerve biopsies, infiltrating leukocytes were positive but class II-positive Schwann cells were not seen. These observations indicate that Schwann cells may not be involved in presenting Mycobacterium leprae antigens to T cells in leprosy. This conflicts with evidence f rom in vitro studies, but may be explained by the fact that in vivo Schwann cells arc surrounded by basement membranes and are closely associated with axons.RÉSUMÉ
Une technique de microscopic électronique a été utilisée pour étudier l'expression de l'histocompatibi-lité majeure de classe II dans des nerfs de lépreux. Dans les nerfs normaux, des cellules endothéliales et d'occasionnelles cellules endoneurales (mais pas des cellules de Schwann) étaient par constitution positives pour la classe II. Dans des biopsies de nerfs de lèpre paucibacillaire et multibacillaire, des leucocytes infiltrants étaient positifs, mais on n'a pas trouvé de cellules de Schwann positives pour la classe IL Ces observations indiquent que les cellules de Schwann pourraient ne pas être impliquées dans la présentation des antigènes de Mycobacterium leprae aux cellules T au cours de la lèpre. Ceci est en contradiction avec ce qui a été vu dans des études in vitro, mais pourrait être expliqué par le fait que les cellules de Schwann sont in vitro entourées par des membranes basales, et sont étroitement associées aux axones.RESUMEN
Se usó una técnica de inmunomicroscopía electrónica para investigar la expresión de antigenos de his-tocompatibilidad clase II en los nervios de casos con lepra. En los nervios normales, la células endotelialcs y las células endoneurales ocasionales (no las células de Schwann) fueron constitutivamente clase II-posi-tivas. En las biopsias de nervios de casos de lepra tanto paucibacilares como multibacilares, los leucocitos infiltrantes fueron positivos pero no se observaron células de Schwann clase II-positivas. Estas observaciones indican que las células de Schwann pueden no estar involucradas en la presentación de antígenos del Mycobacterium leprae a las células T en la lepra. Los hallazgos están en conflicto con los resultados de los estudios in vitro, pero ésto puede explicarse considerando que las células de Schwann in vivo están rodeadas por membranas básales, además de que se encuentran íntimamente asociadas con axones.Mycobacterium leprae has a predilection for living within Schwann cells, and there has been much interest over recent years in whether or not Schwann cells are capable of presenting M. leprae antigens to T lymphocytes in leprosy. Studies on cultured Schwann cells suggest that they may be induced to present antigen under certain circumstances. Samuel, et al. (13,14) have shown that rat and human Schwann cells are able to express major histocompatibility complex (MHC) class II antigens when treated with gamma-interferon in vitro. This is the first requirement for any potential antigen-presenting cell to fulfill before antigen can be recognized by CD4+ T lymphocytes. Wekerle, et al. (16) have shown that such interferon-gamma-stimulated rat Schwann cells are capable of presenting endogenous myelin autoantigens and tuberculin purified protein derivitive (PPD) to antigen-specific T lymphocytes. Kingston, et al. (6) have demonstrated, under somewhat more physiological conditions, that rat Schwann cells can be induced to express class II antigens without pretreatment with interferon-gam-ma if they are cultured together with soluble M. leprae extract and sensitized syngene-ic-T lymphocytes. However, class II induc-ibility seems to be a species-specific phenomenon, since Stcinhoff, et al. (15) have shown that mouse Schwann cells are induced to express class I but not class II antigens by interferon-gamma.
Evidence for antigen presentation by Schwann cells in vivo has been limited to demonstrating class II expression in situ on frozen tissue sections. By this method, several researchers have reported class II-pos-itive cells, presumed by their morphology or by staining serial sections with panels of antibodies to be Schwann cells, in a variety of inflammatory neuropathies (3,7,10,11), including leprosy (9). However, resolution at the light-microscopic level is not sufficient to easily distinguish Schwann cells from fibroblasts and infiltrating cells. Only at the electron-microscopic (EM) level can Schwann cells be clearly identified and intercellular relationships observed. Various immunoelectronmicroscopy (immunoEM) techniques have recently been developed, but the processing schedule required for electron microscopy means that it is very difficult to study labile cell surface proteins such as MHC antigens and CD antigens. This is particularly problematic in neural tissue where strong fixation with glutaral-dehyde and osmium is necessary to preserve the ultrastructure, especially of myelin. There is one report using immunoEM on nerves from Guillain-Barre syndrome patients (10); the technique did not use glutar-aldehyde but involved freezing the tissue, leading to very poor ultrastructure.
Butter, et al. (2) have developed a post-fixation, pre-embedding immunoEM technique which gives clear labeling of leukocyte antigens in neural tissue. This technique was used by Cowley, et al. (4,5) in a guinea-pig model of nerve damage in leprosy, and it was found that although immunostaining of frozen sections suggested that Schwann cells might be class II positive, at the EM level they were negative while infiltrating cells and some fibroblast-like cells were class II positive.
This study uses this immunoEM technique to investigate class II expression in leprosy nerve biopsies in humans.
MATERIALS AND METHODS
Patient Material. Biopsies of the sural or radial cutaneous nerves used for this study were taken from patients attending the All Africa Leprosy and Rehabilitation Training Centre (ALERT), Addis Ababa, Ethiopia. The study was approved by the ALERT/ Armauer Hansen Research Institute (AHRI) Research Committee. Eight biopsies were from new, untreated patients and two were from patients who had been treated many years previously. All patients were undergoing nerve biopsies primarily for diagnostic purposes, usually for suspected neural leprosy where skin biopsies were uninforma-tive. Ten suitable biopsies were obtained in which leprosy was subsequently diagnosed by histological examination of the nerve and where at least some neural tissue remained identifiable (very fibrosed or enormously infiltrated nerves were not used). According to the bacterial index (BI) within the granuloma of the nerve, the nerve lesions were classified as multibacillary (BI > 2, four cases) or paucibacillary (BI < 2, six cases) (8). The BI within the granulomas was assessed in a 5-μm thick section in exactly the same way as in a skin smear. Three tibial nerves from amputations due to vascular disease and one normal radial cutaneous nerve biopsy served as normal control specimens.
Immunoelectronmicroscopy. The nerve biopsies were immediately fixed in cold 1% monomeric glutaraldehyde (Polysciences, U.K.) in Sorensen's reagent for 30 min. They were then supported in 2% agar and 200 μm transverse sections were cut, under fixative, on a Vibroslicer (Campden Instruments, U.K.). After a further 30 min in fixative, the sections were taken through the staining and processing schedule given in The Table. Transverse sections (1 μm) were cut and counterstained with toluidine blue for light microscopy, and suitable areas for ultrathin sectioning were selected. The sections were dried onto 200-mesh copper/rhodium grids and viewed, unpoststained, on an AEI 275 Corinth transmission electron microscope.

Monoclonal antibodies. All of the antibodies used were screened for suitability for this staining technique by preliminary Fac-scan analysis of antibody binding to glutar-aldehyde-fixed human leukocytes (fresh and phytohcmagglutinin-stimulated peripheral blood leukocytes) and also by immuno-staining of human lymphoid tissue at the light- and electron-microscopic level (results not shown). Two monoclonal antibodies specific for MHC class II antigens were selected which were obtained from Biotest (W. Germany): LN3 (mouse IgG2a) reacts with a nonpolymorphic region of the HLA-DR region; Clonab DP/DR (mouse IgG2a) reacts with products of the HLA-DP region and also with the products of the DR region in some haplotypes. Monoclonal antibodies detecting other leukocyte cell-surface antigens were also used: UCHT1 (reacts with the CD3 complex on T cells); 2D1 (detects CD45, "leukocyte common antigen"); SN130 (detects the restricted form of the leukocyte common antigen, CD45R). Appropriate antibody and buffer controls were included in each processing run.
RESULTS
At the light-microscopic level, positively stained cells were identifiable by a brown ring, as with conventional immunoperoxi-dase techniques developed with diamino-benzidine (DAB). Under the electron microscope the reaction product was visible as an electron-dense (i.e., black) deposit on the cell surface. The sections incubated with control reagents were unstained except on the periphery of the block.
In normal, control nerves the only cells staining positively with the anti-class II antibodies were the luminal surface of endothelial cells and occasional endoneurial cells which were not associated with axons and did not have basement membranes (Fig. 1). There was no significant staining of normal nerves with any of the other antibodies.
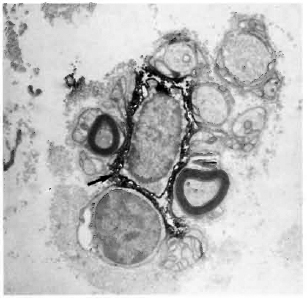
Fig. 1. Electronmicrograph of a normal, human tibial nerve stained with LN3 (antibody against HLA-DR), showing a class II-positive cell (arrow) adjacent to several class II-negative Schwann cells associated with both myelinated and unmyelinated axons ( x 12,000).
In the diseased nerves, T cells and leukocytes were scattered throughout the en-doneurium, as identified using the antibodies UCHT1, 2D1 and SN130 (Figs. 2 and 3). Although the infiltrating cells were found in the general vicinity of the remaining resident Schwann cells and axons, it was very rare to see a lymphocyte in close contact with a Schwann cell. In the multibacillary cases, there was dense collagen surrounding the neural cells which the infiltrating lymphocytes were not often seen to penetrate.
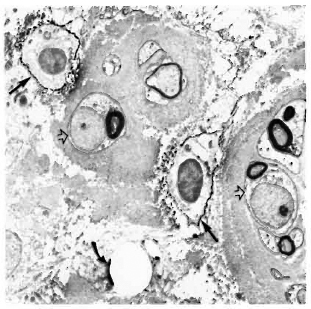
Fig. 2. Multibacillary leprosy nerve biopsy stained with SN130 (antibody against the restricted leukocyte common antigen), showing two positively stained leukocytes (straight arrows) in the vicinity of myelinating Schwann cells which are surrounded by dense collagen (hollow arrows); curved arrow = bacilli ( x4500).
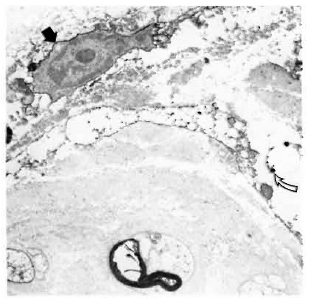
Fig. 3. Multibacillary nerve stained with UCHT1 (antibody against the CD3-complex on T cells), showing a positively stained T cell (straight arrow). Schwann cells arc thickly surrounded by collagen; curved arrow = bacilli ( x7500).
Staining with the anti-class II antibodies showed many positive macrophages (Figs. 4-6), lymphocytes (Fig. 7), and some positive fibroblast-like cells. No class Il-posi-tive Schwann cells, whether associated with myelinated, demyelinated or unmyelinated axons, were seen in any of the multibacillary or paucibacillary leprosy nerves studied (Figs. 4-7). This was not due to inaccessibility of the cells to label, since often a positively labeled mononuclear cell would be in the vicinity of negative Schwann cells (as in Fig. 4).
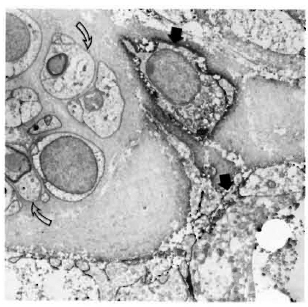
Fig. 4. Multibacillary nerve stained with LN3, showing positive macrophages (straight arrows) and negative Schwann cells (hollow arrows) surrounded by thick collagen ( x7500).
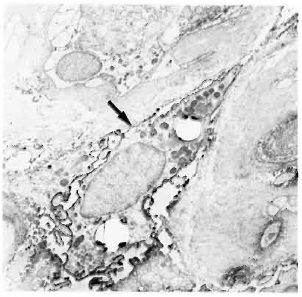
Fig. 5. Class II-positive macrophage containing bacilli (arrow) in a multibacillary nerve biopsy ( x 7500).
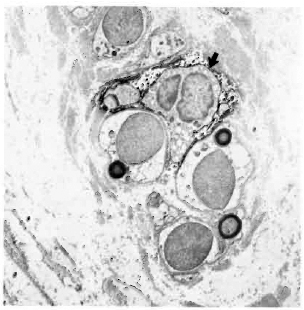
Fig. 6. Paucibacillary nerve stained with LN3, showing a class II-positive macrophage (arrow) among a group of Schwann cells associated with unmyelinated and small myelinated axons ( x 7500).
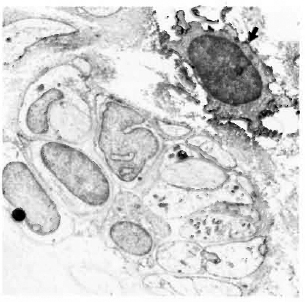
Fig. 7. Class II-positive lymphocyte (arrow) adjacent to Schwann cells and unmyelinated axons in a paucibacillary nerve. There is far less collagen than in the multibacillary nerve biopsies ( x7500).
DISCUSSION
Immunohistochemistry is always a compromise between good tissue preservation and good antigen labeling. In frozen sections, for example, antigen preservation is good but morphological information is very limited. With EM, the range of antibodies that can be used is restricted to those which can detect glutaraldehyde-fixed antigen. The technique we have employed uses relatively weak glutaraldehyde fixation and this, together with the fact that EM sections are not counterstained with uranyl acetate and lead citrate (since this obscures the electron-dense immunostain), means that some definition is sacrificed in the final electronmicro-graphs. Despite these restrictions, immunoEM can yield much more information than standard immunohistochem-istry on frozen tissue. In this and a previous study (4) we have shown that although class II-positive cells in leprosy and experimental granulomatous nerve lesions may appear to be Schwann cells by light microscopy, ultrastructural observations show that Schwann cells, easily identifiable at this level by their basement membranes and/or association with axons, arc class II negative.
This in vivo evidence docs not necessarily conflict with the in vitro studies which demonstrate class II inducibility and antigen presentation by Schwann cells (6,13-16). Schwann cells in culture do not have basement membranes, arc not associated with axons, and do not produce myelin, unlike their well-differentiated in vivo counterparts (1,12). It is possible that only relatively undifferentiated Schwann cells will respond to cytokines by synthesizing class II antigens. This question might be resolved by in vitro studies using organized nerve cultures, where Schwann cells associate with axons and produce basement membranes and myelin (1).
It is also possible that in nerve lesions local levels of cytokines are not great enough to induce class II expression by Schwann cells. Although we have shown that T lymphocytes arc present in areas of remaining Schwann cells, not many T cells were observed in very close proximity to Schwann cells. There was often a thick layer of collagen around the Schwann cells, especially in multibacillary nerves, which may restrict interaction with infiltrating cells and accessibility of cytokines. It is also unknown whether the Schwann-cell basement membrane interferes with immune interactions.
This study has been restricted to cases of pure neuritic leprosy because of ethical considerations when taking nerve biopsies and does not, therefore, represent all cases of leprosy. The immunological events in nerves in early leprosy may differ from those in the established disease where the nerves have become chronically damaged and fibrosed.
Acknowledgments. We are grateful to Dr. Peter Beverley and Dr. Don Healey for the kind provision of monoclonal antibodies. The authors would like to thank Kiros Ayenew, Dr. Bcrhane Kaleab, and Genet Amare for assistance and Dr. Dominique Frommel and Colin Butter for help and discussion. This study was supported by the British Leprosy Relief Association (LEPRA) and was carried out while Sally Cowley was a visiting scientist at AHRI. AHRI is financially supported by the Norwegian and Swedish Agencies for International Development (NORAD and SIDA).
REFERENCES
1. Bunge, R. P., Bunge, M. B. and Eldridge, C. F. Linkage between axonal ensheathment and basal lamina production by Schwann cells. Ann. Rev. Neurosci. 9(1986)305-328.
2. Butter, C, Healey, D, G., Agha, N. and Turk, J. L. An immunoelectronmicroscopical study of class II MHC and a T lymphocyte surface marker during chronic relapsing experimental allergic encephalomyelitis. J. Neuroimmunol. 20(1988)45-51.
3. Cadoni, A., Zicca, A. and Mancardi, G. L. Schwann cell expression of HLA-DR antigen in peripheral neuropathies. (Letter) Lancet 2(1986)1282-1283.
4. Cowley, S. A., Butter, C, Gschmeissner, S. E., Curtis, J. and Turk, J. L. An immunoelectronmicroscopical study of the expression of major histocompatibility complex (MHC) class II antigens in guinea pig sciatic nerves following induction of intraneural mycobacterial granulomas. J. Neuroimmunol. 23(1989)223-231.
5. Cowley, S. A., Butter, C, Verghese, S., Curtis, J. and Turk, J. L. Nerve damage induced by mycobacterial granulomas in guinea pig sciatic nerves. Int. j. Lepr. 56(1988)283-290.
6. Kingston, A. E., Bergsteinsdottir. K., Jessen, K. R., van der Meide, P. H., Colston, M. J. and Mirsky, R. Schwann cells co-cultured with stimulated T cells and antigen express major histocompatibility complex (MHC) class II determinants without interfcron-γ pretreatment: synergistic effects of interfcron-γ and tumor necrosis factor on MHC class II induction. Eur. J. Immunol. 19(1989)177-183.
7. Mancardi, G. L., Cadoni, A., Zicca, A., Schenone, A., Tabaton, M., De Martini, I. and Zaccheo, D. HLA-DR Schwann cell reactivity in peripheral neuropathies of different origins. Neurology 38(1988)848-851.
8. Negesse, Y. Leprous neuritis, classification of leprosy and multidrug therapy. (Letter) Int. J. Lepr. 56(1988)329-330.
9. Nilsen, R., Mshana, R. N., Negesse, Y., Mengistu, G. and Kana, B. Immunohistochem-ical studies of leprous neuritis. Lepr. Rev. 57Suppl.2(1986)177-187.
10. Pollard, J. D., Baverstock, J. and McLeod, J. G. Class II antigen expression and inflammatory' cells in the Guillain-Barré syndrome. Ann. Neurol. 21(1987)337-341.
11. Pollard, J. D., McComhe, P. A., Baverstock, J., Gatenby, P. A. and McLeod, J. G. Class II antigen expression and T lymphocyte subsets in chronic inflammatory demyclinating polyneuropathy. J. Neuroimmunol. 13(1986)123-134.
12. Samuel, N. M., Crawford, C. L. and Grange, J. M. Ultrastructure of human foetal Schwann cells in tissue culture infected with Mycobacterium leprae. Lepr. Rev. 59(1988)17-24.
13. Samuel, N. M., Jessen, K. R., Grange, J. M. and Mirsky, R. Gamma interferon, but not Mycobacterium leprae, induces major histocompatibility class II antigens on cultured rat Schwann cells. J. Neurocytol. 16(1987)281-287.
14. Samuel, N. M., Mirsky, R., Grange, J. M. and Jessen, K. R. Expression of major histocompatibility complex class I and class II antigens in human Schwann cell cultures and effects of infection with Mycobacterium leprae. Clin. Exp. Immunol. 63(1987)500-509.
15. Steinhoff, U. and Kaufmann, S. H. E. Specific lysis by CD8+ T cells of Schwann cells expressing Mycobacterium leprae antigens. Eur. J. Immunol. 18(1988)969-972.
16. Wekerle, H., Schwab, M., Linington, C. and Meyermann, R. Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur. J. Immunol. 16(1986)1551-1557.
1. Ph.D.; Department of Pathology, Royal College of Surgeons of England, 3543 Lincoln's Inn Fields, London WC2A 3PN, U.K.
2. B.Sc; Department of Pathology, Royal College of Surgeons of England, 3543 Lincoln's Inn Fields, London WC2A 3PN, U.K.
3. Ph.D., Department of Pathology, Royal College of Surgeons of England, 3543 Lincoln's Inn Fields, London WC2A 3PN, U.K.
4. M.D., Department of Pathology, Royal College of Surgeons of England, 3543 Lincoln's Inn Fields, London WC2A 3PN, U.K.
5. M.D., Armauer Hansen Research Institute (AHRI) and All-Africa Leprosy & Rehabilitation Training Centre (ALERT), Addis Ababa, Ethiopia.
Received for publication on 20 October 1989.
Accepted for publication on 26 January 1990.