- Volume 57 , Number 2
- Page: 554–6
HLA-DQ molecules may be products of an immune suppression gene responsible for Mycobacterium leprae-specific nonresponsiveness
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
To the editor:
HLA-DQwl was found to be associated with lepromatous leprosy in several populations (3,12). The increasing evidence for suppressor-T (Ts) cells in lepromatous leprosy (1,8,10) suggests that this association and the HLA-linked control of susceptibility to lepromatous leprosy may be due to an immune suppression (Is) gene (2,9,11). Based on the observation that helper-T-cell responsiveness could be restored by anti-DQ antibodies, Sasazuki and his co-workers have put forward the hypothesis that DQwl molecules may be the products of an HLADQwl-associated Is gene, which might also be the explanation for the association between DQwl and lepromatous leprosy (5,11). To test this hypothesis, we studied 18 lepromatous leprosy patients who were nonresponsive to Mycobacterium leprae antigens.
Proliferative assays. Peripheral blood mononuclear cells (PBMC) of 18 lepromatous leprosy patients were isolated as described previously (4). The diagnosis of all patients used for this study was based on regular clinical examination, lepromin skin test, and skin-biopsy histology by Dr. D. L. Leiker, Department of Dermatology, University Hospital of Amsterdam, Amsterdam, The Netherlands. A standard lymphocyte proliferation assay was performed in which the soluble M. leprae preparations CD67.11 (kindly provided by Dr. R. J. W. Rees, IMMLEP M. leprae bank, London; 2.5 μg/ml) or Dharmendra lepromin (Dh. 1: 60) (kindly provided by Dr. R. C. Good, Centers for Disease Control, Atlanta, Georgia, U.S.A.) were added to 10s PBMC in flat-bottomed, 96-well, microtiter plates (Greiner, Federal Republic of Germany). After incubation in a fully humidified 5% CO2-air mixture for 5 days at 37ºC, 1.0 μCi 3H-thymidine (3H-TdR) was added to each well. After a further 16 hr, the cultures were collected on glass-fiber filters using a semiautomatic sample harvester, and 3H-TdR incorporation was counted by liquid scintillation.
Monoclonal antibodies (mAbs). The murine mAbs used in this study were B9.12.1 (anti-HLA class I monomorphic, IgG2A, gift of B. Malissen); B8.11.2 (anti-DR monomorphic, IgG2b, gift of B. Malissen); SPVL3 (anti-DQ monomorphic, IgG2a, gift of H. Spits); IIB3 (anti-DQw 1, DQw4, IgG2b, gift of F. Koning); IVD-12 (anti-DQw3, IgGl, gift of R. C. Giles); HU-11 (anti-DQw 1, gift of M. Aizawa); and HU-18 (anti-DQw3, IgG2b, gift of M. Aizawa). All mAbs consisted of mouse-derived ascites. All mAbs are described in reference 7 except for HU11 and HU-18 (see 6).
Restoration of PBMC's proliferative immune response by mAb. At the start of the culture described above, 0.05 ml Iscove's modified Dulbecco's medium (IMDM) with mAb was added. All mAbs were filter-sterilized through 0.22-μm filters (Gelman Instrument Co., Ann Arbor, Michigan, U.S.A.), and tested in a concentration of 1:200.
All PBMC from 18 lepromatous leprosy patients were unresponsive to M. leprae per se (left two rows in The Table). To test whether this antigen-specific unresponsiveness is regulated via an HLA Is gene product, we performed blocking studies using various mAbs against HLA antigens. Whole PBMCs were cultured with or without M. leprae antigen in the presence of various mAbs for 6 days. As shown in The Figure, such cells from patient W2 showed a significant response when mAb against HLADQ (SPVL3) was added at the start of culture but the response could not be restored by mAbs against HLA class I, DR (The Figure), DQw3 (mAb IVD-12), and two DQw 1 mAbs-Hu-11 and IIB3 (data not shown). The results were repeatablc, that is, the M. leprae-specific response could be restored in only 1 out of 18 lepromatous leprosy patients.
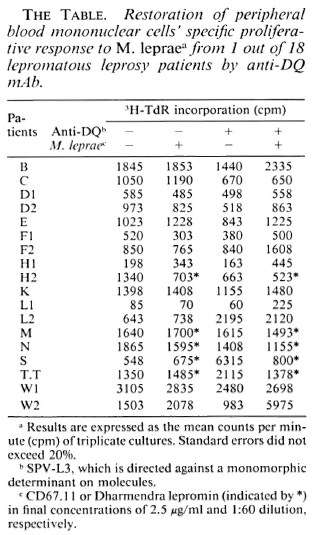
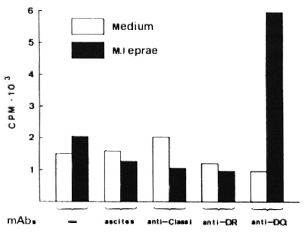
The Figure. Restoration of PBMC's proliferative immune response to M. leprae by anti-DQ mAb from one nonresponder lepromatous leprosy patient. PBMC were in a concentration of 105 cells per well. Results are expressed as the mean cpm x 10-3 of triplicate cultures (3H-thymidine incorporation). Standard errors did not exceed 20%. Ascites was SPO2; mAbs are described in text.
We and others (T. Sasazuki and T. Ottenhoff, personal communications) have observed that anti-DQ mAb could abolish M. leprae-specific suppression, indicating that HLA-DQwl may be the product of an Is gene for M. leprae. We observed restoration of the response only with mAb SPVL3 (reactive with a nonpolymorphic determinant on DQ molecules) and not for anti-DQwl mAbs (HU-11 and IIB3, data not shown). This could be due to the polymorphism of DQ molecules (DQ typing of patient W2 is DQw3 and DQw7) and to the different specificities/affinities of the anti-DQ mAbs. However, because the phenomenon was observed so infrequently, it seems unlikely to us that this observation can explain the association between HLA-DQwl and lepromatous leprosy. Therefore, we have undertaken a more complete study of the restriction and regulation by HLA products of M. leprae-specific suppressor-T-cell clones.
- Shu-Guang Li, M.D.
- René R. P. de Vries, M.D., Ph.D.
Department of Immunohematology and Blood Bank
University Hospital
Rijnsburgerweg 10
2333 AA Leiden, The Netherlands
Acknowledgments. We would like to thank Dr. Frits Koning and Dr. Takehiko Sasazuki for providing the monoclonal antibodies, Dienne Elferink for technical assistance. Dr. Tom Ottenhoff for helpful discussions, Mrs. Jane Thorogood for reading the manuscript, and Ellen van der Willik-van Harteveld, Ingrid Curiel and Tiny van Westerop for preparation of the manuscript. Financial support for this study was obtained from the Immunology of Leprosy (IMMLEP) component of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, The Netherland Leprosy Relief Association (NSL), the Dutch Foundation for Medical Research (MEDIGON Grant no. 900-509-099), and the J. A. Cohen Institute for Radiopathology and Radiation Protection (IRS).
REFERENCES
1. BLOOM, B. R. and MEHRA, V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80(1984)5-28.
2. DE VRIES, R. R. P., OTTENHOFF, T. H. M. and VAN SOIOOTEN, W. C. A. Human leucocyte antigens(HLA)and mycobacterial disease. Springer Semin. Immunopathol. 10(1988)305-318.
3. DE VRIES. R. R. P., SERJEANTSON, S. W. and LAYRISSE, Z. Leprosy. In: Histocompatibility Testing 1984. Albert. E. D., Baur, M. P. and Mayer, W. R., eds. Berlin: Springer Verlag, 1984, pp. 362-367.
4. HAANEN, J. B. A. G., OTTENHOFF, T. H. M., VOORDOUW, A., ELFERINK, D. G., KLATSER, P. R., SPILTS, H. and DE VRIES, R. R. P. HLA class II restricted Mycobacterium leprae reactive T cell clones from leprosy patients established with a minimal requirement for autologous mononuclear cells. Scand. J. Immunol. 23(1986)101-108.
5. HIRAYAMA, K., MATSUSHITA, S., KIKUCHI, I., IUCHI, M., OIITA, N. and SASAZUKI, T. HLA-DQ is epistatic to HLA-DR in controlling the immune response to schistosomal antigen in humans. Nature 327(1987)426-430.
6. IKEDA, H., KASAIIARA, M., OGASAWARA, K., TAKENOUCHI, T., OKUYAMA, T., ISHIKAWA N., WAKISAKA. A., KIKUCHI, Y. and AIZAWA, M. Evidence for polymorphism of MB3 antigens among three HLA-D clusters associated with HLA-DR4. Immunogenetics 19(1984)381-390.
7. KONING, F. Identification and functional relevance of epitopes on human lymphocytes, thesis. University of Leiden, 1986.
8. MODLIN. R. L., KATO, H., MEHRA, V., NELSON, E. E., FAN, X.-O., REA, T. H., PATTENGALE, P. K. and BLOOM, B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. Nature 322(1986)459-461.
9. OTTENHOFF, T. H. M. and HE VRIES. R. R. P. HLA class II immune response and suppression genes in leprosy. Int. J. Lepr. 55(1987)521-534.
10. OTTENHOFF. T. H. M., ELFERINK, D. G., KLATSER, P. R. and DE VRIES, R. R. P. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. Nalure 322(1986)462-464.
11. SASAZUKI, T., NISHIMURA, Y., MUTO, M. and OHTA, N. HLA-linked genes controlling immune response and disease susceptibility. Immunol. Rev. 70(1983)51-75.
12. SERJEANTSON, S. W. HLA and susceptibility to leprosy. Immunol. Rev. 70(1983)89-112.