- Volume 58 , Number 1
- Page: 1–11
Joint Chemotherapy trials in lepromatous leprosy conducted in Thailand, the Philippines, and Korea
ABSTRACT
Chemotherapy trials in lepromatous leprosy using various combinations of existing antileprosy drugs were conducted jointly by Korea, The Philippines, and Thailand. The general objective of these trials was to determine the most effective and practicable regimen or regimens for field application.
Lepromatous patients were divided into two groups: Group I was comprised of new, untreated patients infected with dapsonesensitive Mycobacterium leprae and Group II consisted of relapsed patients with dapsone-resistant disease. Four different regimens were administered to each group for 5 years. Comparison among the regimens was based on antileprotic efficacy, drug safety, acceptability, field practicability, and economic feasibility.
No significant differences were noted among the various regimens as judged by the reduction in the bacterial index (BI), clinical response, and change in biopsy index. Toxicity was seen only in the regimens containing prothionamide and rifampin. The regimens were acceptable to the patients and all were found practical for field use. Clofazimine, even in low doses, was found to suppress the frequency and severity of erythema nodosum leprosum. A multidrug regimen effective against both new and relapsed cases of lepromatous leprosy, whether dapsone sensitive or dapsone resistant, is recommended for field use. Given priority, the cost of the regimens is affordable in the three countries.
RÉSUMÉ
On a mené en Corée, aux Philippines et en Thailande, des essais de chimiothérapie chez des malades lépromateux, au moyen de diverses combinaisons de médicaments anti-lépreux existants. L'objectif général de ces essais était de déterminer les posologies les plus efficaces et les mieux adaptées pour l'application sur le terrain.
Les malades lépromateux ont été divisés en deux groupes. Le groupe I comprenait des malades nouveaux et non traités, infectés par des souches de Mycobacterium leprae sensibles à la dapsone; le groupe II consistait en malades ayant récidivé et présentant une affection résistante à la dapsone. Quatre posologies différentes ont été administrées à chacun de ces deux groupes pendant 5 ans. La comparaison entre les différentes posologies a pris en compte l'efficacité contre la lèpre, la tolérance au médicament, l'acceptabilité, la facilité d'application sur le terrain, et les contraintes économiques.
Aucune différence significative n'a été notée entre les diverses posologies pour autant que l'on puisse en juger par la réduction de l'index bactériologique (BI), par la réponse clinique, et par les modifications notées dans l'index biopsique. C'est seulement avec des posologies contenant de la prothionamide et de la rifampicine que l'on a constaté des effets toxiques. Les posologies utilisées étaient acceptables pour les malades. Toutes se sont révélées pratiques quant à l'application sur le terrain. On a observé que la clofazimine, même à doses faibles, réduisait la fréquence et diminuait la gravité de l'érythème noueux lépreux. On recommande dès lors d'utiliser sur le terrain une posologie polychimiothérapique qui soit efficace tant chez les nouveaux cas que chez les récidives de lèpre lépromateuse, sans égard pour la sensibilité à la dapsone. Compte tenu de la priorité du problème, ces diverses posologies ne sont pas trop coûteuses dans les trois pays considérés.
RESUMEN
Se realizó un programa de tratamiento conjunto, con varias combinaciones de drogas antileprosas, en Corea, Filipinas y Tailandia. El objetivo general del ensayo fue el determinar el esquema de tratamiento más efectivo para su aplicación en el campo. Los pacientes lepromatosos se dividieron en 2 grupos: el grupo I estuvo constituido por pacientes no tratados, infectados con Mycobacterium leprae sensible a la dapsona; el grupo II consistió de pacientes recurrentes, con enfermedad resistente a la dapsona. A cada grupo se le administraron 4 esquemas diferentes de tratamiento durante 5 años. La comparación entre los esquemas de tratamiento se basó en la eficacia antileprótica, la seguridad de las drogas, la aceptabilidad, la practicabilidad en el campo, y la factibilidad económica.
A juzgar por la reducción en el índice bacteriano (IB), la respuesta clínica, y el cambio en la biopsia, no se notaron diferencias significativas entre los diversos esquemas utilizados. Solo se vio cierta toxicidad en el esquema que contenía protionamida y rifampina. Los esquemas de tratamientos fueron aceptables por los pacientes y todos resultaron prácticos para su uso en el campo. La clofazimina, aún en dosis bajas, suprimió la frecuencia y severidad del eritema nodoso leproso. Para su uso en el campo se recomienda un esquema con múltiples drogas contra casos nuevos y recurrentes de lepra, ya sean sensibles o resistentes a la dapsona. El costo de los esquemas de tratamiento está dentro de las posibilidades de los 3 países involucrados.
Dapsone monotherapy was extensively used for the treatment of leprosy from the early 1950s until the late 1970s. However, when the ever-increasing threat of dapsone resistance began to jeopardize the control of the disease worldwide (7,8,11) various attempts were made by different antileprosy agencies to counter the threat. In January 1977, the Sasakawa Memorial Health Foundation of Tokyo, Japan, organized The 1st International Workshop on Chemotherapy of Leprosy in Asia, held in Manila, The Philippines (10). They recognized the necessity of adopting combined chemotherapy in the treatment of multibacillary leprosy to curtail the problem of drug resistance (4,5,16,18). The rationale was that Mycobacterium leprae resistant to one of the drugs used in the treatment would be killed by the other drugs in the combination, thus preventing the emergence of drug resistance as a clinical problem.
The International Symposium on Leprosy and Joint Chemotherapy Trial Meeting (2) in Seoul and Anyang, Korea, in 1978, organized by the Korean Leprosy Control Association and supported by the Sasakawa Memorial Health Foundation of Japan, discussed the problem of drug resistance and its grave consequence to leprosy control programs worldwide. As a result of this meeting, the health authorities of Thailand, The Philippines, and Korea organized and implemented the Joint Chemotherapy Trials in Lepromatous Leprosy (JCT), using various combinations of existing antileprosy drugs (3). The trials spanned 9 years, starting in 1979 and ending in 1988.
The broad objective of the trials was to select the most effective and practicable combined therapy regimen or regimens for field application to be recommended to the governments of each country. The specific objective was to compare the different regimens used as to their antileprosy efficacy, drug safety, acceptability, field practicability, and economic feasibility (2,10).
MATERIALS AND METHODS
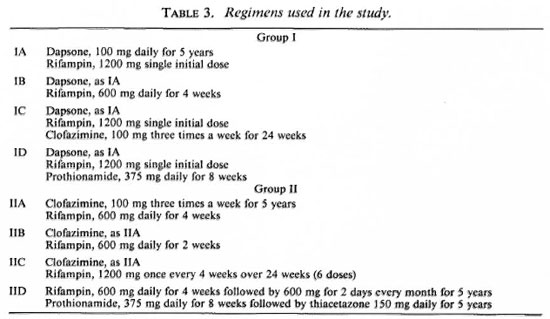
Study population. Lepromatous patients, new and relapsed, male and female, 15 to 60 years of age, with an average bacterial index (BI)ofat least 4+ were recruited over a period of 4 years. A medical examination that included hematology, 'blood chemistries, and chest X-rays, was used as a screen to exclude intercurrent life-shortening diseases. Those patients found suitable were biopsied for a histopathologic evaluation (12) and a mouse foot-pad inoculation test to determine dapsone sensitivity using standard techniques (15). The patients were then divided into two main groups: Group I consisted of new patients; Group II was composed of relapsed patients previously treated with dapsone as a monotherapy. Group I patients were randomized in each country into two subgroups and designated as Groups IA and IB in Thailand, IA and IC in The Philippines, and IA and ID in Korea. Similarly, Group II patients were randomized into two subgroups in each country and designated as Groups IIA and IIB in Thailand, IIA and IIC in The Philippines, and IIA and IID in Korea. Different regimens were assigned to each subgroup, but regimens IA and IIA were the same in all three countries. Treatment was then given to all patients while waiting for the drugsensitivity tests in mice. Only those Group I patients found to be dapsone sensitive and Group II patients found to be dapsone resistant were finally entered into the study. The other patients were not included in the trial, and their treatment was changed to the usual regimen of the country concerned.
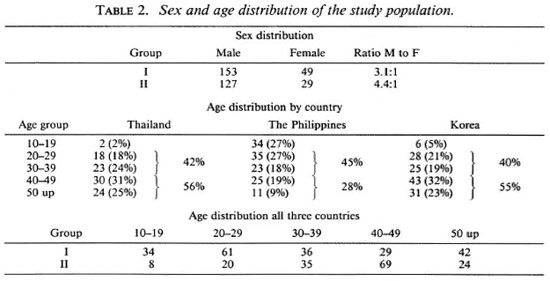
Table 1 shows that a total of 358 patients were entered into the study in the three participating countries-97 in Thailand, 128 in The Philippines, and 133 in Korea. Table 1 also shows the number of patients in the different regimens in each of the three countries
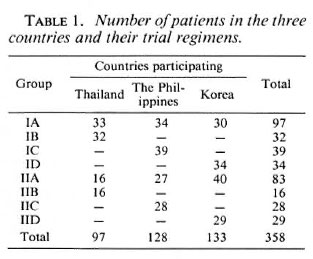
Table 2 shows the sex and age distribution of the patients in the study. There were 3.1 males to 1 female in Group I patients and 4.4 males to 1 female in Group II patients. The patients in Thailand and Korea were older than those in The Philippines. Patients over 40 years of age made up 56% of the patients in Thailand; in Korea, 55%; in The Philippines, only 28%. The age group 20-29 years is practically the same for all three countries; 42% in Thailand, 45% in The Philippines, and 40% in Korea. In the age group 10-19 years, Thailand had only 2% of their patients; Korea had 5%; The Philippines, 27%. In the three countries, more Group II patients were in the older age bracket than Group I. This is understandable, since Group II patients were the relapsed cases and had had the disease longer than the Group I patients.
Regimens used. Table 3 shows the different regimens used in the study with each regimen used for 5 years duration.
Study phases. The trials were implemented in three phases. The first phase (3 months) consisted of admission to one of the trial centers for initial screening tests, initiation of therapy, and observation for early signs of drug toxicity and side effects. In phase 2 (3 months to 2 years), all patients were routinely treated and examined mostly as outpatients. Phase 3 was a follow-up period of 3 years, during which the patients were observed for further clinical and bacteriological improvement and for possible signs of reactivation.
Periodic examinations. Periodic examinations were done at 0, 6, 12, 18, 24, 36, 48 and 60 months. These included a clinical examination, lepromin test, photography, dapsone (DDS) in urine, skin smears, nose blows, histopathology, mouse foot-pad testing, and general laboratory examinations of urine and blood to monitor toxicity.
Exclusion during the trials. Patients were removed for drug toxicity, worsening of the disease, side effects which required alteration of the assigned regimen, severe erythema nodosum leprosum (ENL), serious intercurrent diseases, or on voluntary patient withdrawal.
RESULTS AND DISCUSSION
The antileprosy efficacy of the different regimens used in the trials was judged by changes in the BI rate of clinical improvement and histopathological changes. Drug safety was judged by the frequency and severity of toxicity and frequency and severity of ENL. Acceptability of the regimens was determined by the severity of side effects and patient compliance with prescribed drugs. Field practicability was judged by the ease, frequency, and duration of drug administration; length of shelf life and ease of storage of drugs under varying local conditions. Economic feasibility was judged in terms of the cost of the different regimens.
Changes in BI. Figure 1 shows the changes in the average BI at different periods of therapy for patients in Group 1A, a common regimen in all three countries. The fall in BI is approximately the same in all three countries, and no significant statistical differences between regimens was noted. The curves tend to show that new, untreated lepromatous patients sensitive to dapsone in the three countries respond in the same way to a regimen of daily dapsone 100 mg for 5 years and a single initial dose of 1200 mg rifampin as far as the fall in the BI is concerned. Because of this nearly uniform response, a common curve representing the Group IA average of the three countries was calculated and placed as the fourth curve in the figure. This common curve was used as a control to compare with Regimens IB, IC, and ID of the group. It is also noted in the figure that the fall in the BI of all Group IA regimens did not reach zero at the end of 5 years.
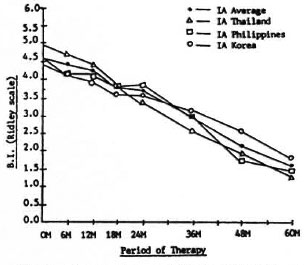
Fig. 1. Changes in bacterial index (BI) of Group 1A in all countries.
Figure 2 shows the changes in the average BI at different periods of therapy for patients in Group IIA, also a common regimen in all three countries. It shows that the fall in the BI is approximately the same in the three countries, and no significant statistical differences among the regimens was observed. The curves also tend to show that previously treated, relapsed patients with dapsone-resistant infection in the three countries responded in the same way to a regimen of clofazimine 100 mg three times a week for 5 years and rifampin 600 mg daily for 4 weeks. Again, because of the almost uniform response of Group IIA patients, a common curve was calculated to represent the IIA average of the three countries (Fig. 2). This common curve was used as a control to compare with Regimens IIB, IIC and IID of the group.
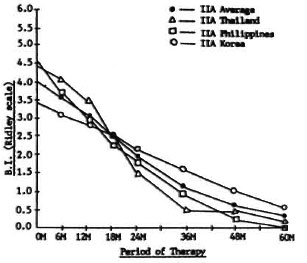
Fig. 2. Changes in bacterial index (BI) of Group IIA in all countries.
It can be noted that the fall in the BI of Group IIA (Fig. 2) nearly reached 0 in 5 years, and the rate of fall is much faster compared to that of Group IA patients (Fig. 1). However, there is no clear evidence in the study to prove that the difference was the result of Regimen IIA being superior to IA. It is more probable that the difference is due to the presence of histoid and other peculiar lesions commonly found in the skin of relapsed patients. These lesions are irregularly distributed and contain high local concentrations of bacilli (14). The initial skin smears are usually taken from these sites resulting in a report of a high BI. These lesions, however, will respond more quickly to adequate treatment and, thus, a faster fall in the BI is observed in succesive smears.
Figure 3 shows the changes in the BI of Groups IB, IC, and ID patients compared to the IA average of the three countries used as a control. All Group I regimens were basically a dapsone/rifampin combination. Regimen IB had 4 weeks of daily rifampin. To Regimen IC was added clofazimine 100 mg three times a week for 24 weeks, and to Regimen ID was added prothionamide 375 mg daily for 8 weeks. In spite of the additional rifampin in Regimen IB, clofazimine in Regimen IC and prothionamide in Regimen ID, the fall in the BI is the same as with Regimen IA, which is only a combination of daily dapsone and a single dose (1200 mg) of rifampin. No statistically significant differences among regimens were noted. It would seem that one initial dose of 1200 mg rifampin added to 100 mg dapsone given daily for 5 years is just as effective as a 5-year dapsone/rifampin combination with more rifampin given or with clofazimine or prothionamide added.
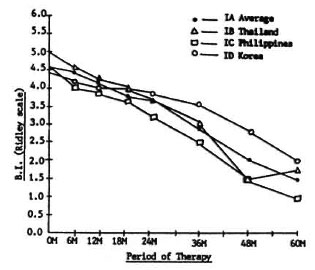
Fig. 3. Changes in bacterial index (BI) of Group I in all countries.
It is now known that the combination of rifampin and prothionamide is potentially toxic (1). In Regimen ID, however, where both drugs were combined, rifampin was given only once as a single initial dose of 1200 mg and no toxic effect was observed.
Figure 4 shows the changes in the BI of Groups IIB, IIC, and IID patients compared to the IIA average of the three countries used as a control. Regimens IIA, IIB, and IIC were all clofazimine/rifampin combinations with clofazimine given as the basic drug for 5 years. Only the dose of rifampin varied. In Regimen IIA rifampin was given 600 mg daily for 4 weeks; in IIB, 600 mg daily for 2 weeks; in IIC, 1200 mg once every 4 weeks for 24 weeks (six doses). Regimen IID was different in that it was a rifampin/prothionamide/thiacetazone combination. Figure 4 shows that the fall in the BI is the same for all regimens, and no statistically significant differences exist. Thus, increasing the dosage of rifampin or a combination of rifampin/prothionamide/thiacetazone is no more effective than rifampin 600 mg daily for 2 weeks (Regimen IIB) or rifampin 1200 mg once every 4 weeks for 24 weeks (Regimen IIC).
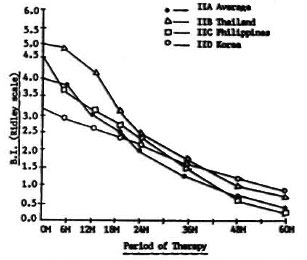
Fig. 4. Changes in bacterial index (BI) of Group II in all countries.
As has already been stated, a rifampin/ prothionamide combination is potentially toxic. In Regimen IID, where both drugs were combined in the initial 8 weeks of treatment, there was a rise in SGPT in 40% of the patients in that group, with two cases of overt jaundice necessitating removal from the study.
Nose blows. Nasal discharge, especially in the lepromatous type of the disease, is important to public health because of strong evidence that the respiratory tract is the portal of exit of M. leprae (9) and a likely factor in the spread of the disease. In this study, nose blows were examined for acid-fast bacilli (AFB) before treatment and at 1, 2, and 3 years after the start of therapy.
For Group I patients, the results were the same for all regimens, and so no comparison was possible. The initial percentage of patients with positive nose blows in this group was in the range of 60% to 75%, falling after therapy to 20% to 25% at 1 year, 10% at 2 years, and 0% at 3 years.
For Group II patients, the results for all the regimens were also the same, and no comparison was possible. It was noted that initially the percentage of positive nose blows in this group was only 30% to 40%, much lower than in Group I patients, then falling rapidly to 10% to 20% at 1 year, 0% to 10% at 2 years, and 0% at 3 years. Being relapsed and previously treated patients, the comparatively small number of positive nose blows may have been due to their previous treatment which strengthened the integrity of their nasal mucosae.
Rate of clinical improvement. Clinical assessment was made in accordance with the standard THELEP protocol (17). The findings at different periods of therapy were compared to the findings at initial examination and the difference was graded as: deterioration, no change, slight improvement, or definite improvement.
Table 4 shows the clinical assessment for Group I patients. The findings at different periods of therapy are approximately the same for all regimens in the group, and no statistically significant differences exist among them. It can generally be said that if we were to lump together all degrees of improvement (slight and definite), about 78% to 94% of all Group I patients clinically improved in 6 months and from about 92% to 100% in 12 months. From then until 5 years, the percentage of patients who improved clinically was maintained at nearly 100% to 100%. It will be noted that the onset of clinical improvement begins very early in the treatment period and is sustained at a high percentage level in 2 years time.
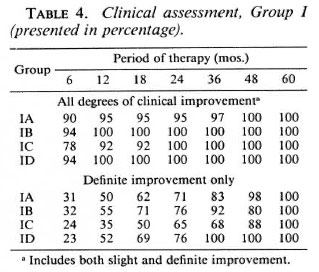
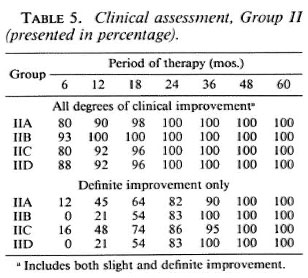
Table 5 shows the clinical assessment for Group II patients. The findings at different periods of therapy are also the same for all the regimens in the group, and no statistically significant differences among regimens were seen. Considering all degrees of improvement (slight and definite), about 80% to 93% of all Group II patients improved in 6 months; about 90% to 100% in 12 months. From that time until 5 years, nearly 100% to 100% of the patients improved. The table shows that even in the group of relapsed and dapsone-resistant patients, clinical improvement began very early in the course of treatment as in new and untreated patients.
Histopathologic changes. It was attempted in this study to show the changes in the biopsy indices (13) of the different regimens at different periods of therapy. Figure 5 shows the percent improvement of the biopsy indices of Groups IA, IB, and IC, with IA being the average of Thailand and The Philippines used as a control. The percent improvement at different periods of therapy was more or less the same for the three regimens. No statistically significant difference among regimens was observed. Improvement generally increased in the three regimens to about 40% to 50% in 12 months, 60% to 65% in 24 months, 70% to 80% in 36 months, and 80% to 90% in 48 months to 5 years. This finding correlates well with the fall in the BI and clinical improvement.
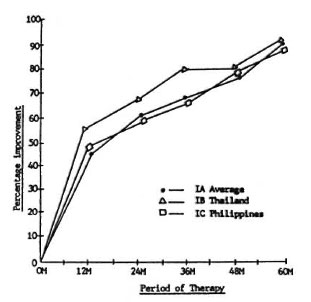
Fig. 5. Biopsy index percentage improvement, Group I.
Figure 6 shows the percentage improvement of the biopsy indices of Groups IIA, IIB, and IIC, with IIA being the average of Thailand and The Philippines used as a control. The degree of improvement was about the same in the three regimens, and no significant statistical differences were seen. Also, the same good correlation with the fall in the BI and clinical improvement is noted in this group.
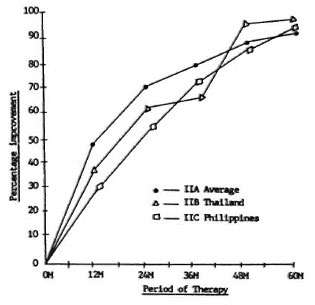
Fig. 6. Biopsy index percentage improvement, Group II.
Frequency of ENL. Severity of ENL was graded in accordance with the standard THELEP protocol for chemotherapeutic studies: 14- (mild), 2+ (moderate) and 3 + (severe). Table 6 shows the frequency and severity of ENL in Group I patients in all three countries. Considering moderate to severe ENL (2+ and 3+ ENL only), the range is only 3%-14% at 6 months, about 20% at 12-36 months, about 10%-30% at 48 months and 0% at 60 months.
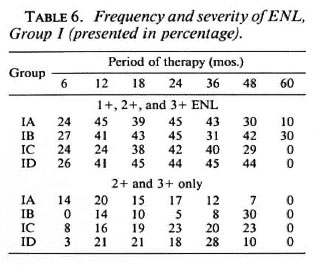
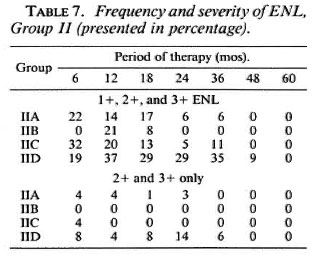
Table 7 shows the frequency and severity of ENL in Group II patients of all three countries. The overall frequency and severity of ENL is much less in this group compared to Group I patients. Group I regimens do not contain clofazimine, except IC in its first 24 weeks of treatment. Regimens IIA, IIB, and IIC contain clofazimine given for 5 years; IID does not but is a rifampin/ prothionamide/thiacetazone combination. Table 7 shows that among Group II patients, those taking Regimens IIA, IIB, and IIC have a much less percentage frequency and severity of ENL than those taking Regimen IID. This finding shows that clofazimine, even in low doses, can suppress the frequency and severity of ENL.
Toxicity. Toxicity was detected only in Regimen IID, containing prothionamide combined with rifampin and given for the first 8 weeks of treatment. This produced a rise in SGPT in 40% of the patients in that regimen. The SGPT levels, however, returned to normal after prothionamide was stopped at 8 weeks. This should alert clinicians to possible liver damage in the use of prothionamide in combination with rifampin, and this combination should not be recommended for field use.
Acceptability of the different regimens. Acceptability of the different regimens was generally good except for those regimens containing clofazimine, which produced pigmentation (6) and presented some difficulty to light-skin patients, particularly when treated as outpatients. However, with proper motivation emphasizing the importance of the drug in the combination, the patient usually accepted it.
Field practicability. The different regimens were generally found to be practicable for use under field conditions in all three countries. The length of shelf life and ease of storage of the drugs under varying local conditions were met satisfactorily except for clofazimine, which has a tendency to melt under severe tropical heat and humidity and to stick together making separation difficult and messy. However, putting some inert powder in the container with the clofazimine prevents the capsules from sticking together.
Mouse inoculation tests. The mouse footpad tests for dapsone resistance were done on all patients in all three countries. The new and untreated patients who were candidates for Group I came into the study sequentially and randomly. For this reason, the primary dapsone-resistance figures from the mouse foot-pad test results from these patients may be suggestive of the true prevalence of primary dapsone resistance in each of the three countries. Thailand reported a prevalence of 22.4% (15% low, 6% moderate, and 1.4% high). Korea had a prevalence of 20.5% (12.8% low, 2.6% moderate, and 5.1% high). The Philippines had a prevalence of only 6.1% (4.6% low, 1.5% moderate, and 0% high). Primary dapsone resistance in the three countries is more of the low-resistance type. A large disparity exists in the prevalence of primary dapsone resistance between Korea and Thailand on one hand, and The Philippines on the other. This requires further study.
The previously treated and relapsed patients who were candidates for Group II were clinically selected on the basis of likelihood of fitting into the group. Thus, the results of the mouse foot-pad drug-sensitivity tests in these patients cannot be interpreted as the true prevalence of secondary dapsone resistance in the three countries. However, the figures are given here for the purpose of record. Of those tested in Thailand, 73.3% were found to be dapsone resistant (26.6% low, 20.1% moderate, and 26.6% high). In Korea, 78.4% were dapsone resistant (10.8% low, 29.8% moderate, and 37.8% high). In The Philippines, 69.4% were dapsone resistant (11.8% low, 28.2% moderate, and 29.4% high).
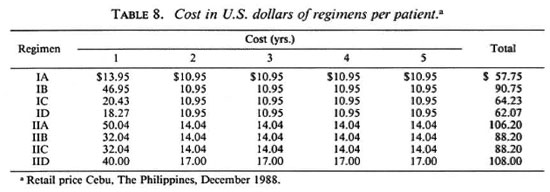
Cost of the different regimens. Table 8 shows the cost of the different regimens. The costs were highest in those combinations containing the most rifampin, as high as US$ 108.00 per patient for 5 years, and lowest in those regimens containing only daily dapsone and a single dose of rifampin, which was US$57.75 per patient for 5 years.
CONCLUSIONS
1. No significant differences were found among regimens in Group I and Group II as judged by the fall in the BI, clinical assessment, and changes in the biopsy index. The three parameters correlate with each other in a general way. In new untreated cases of lepromatous leprosy, a regimen containing one initial dose of 1200 mg rifampin added to 100 mg daily dapsone for 5 years was just as effective compared to 5-year dapsone/rifampin regimens where more rifampin was given or where clofazimine or prothionamide was added. In relapsed cases, giving rifampin 600 mg daily for 2 weeks or rifampin 1200 mg every 4 weeks for 24 weeks (six doses) and clofazimine 100 mg three times a week for 5 years was just as effective as regimens with more rifampin or to one regimen with prothionamide and thiacetazone added. Clinical improvement began very early in the course of treatment in both the new and relapsed patients.
On the basis of this study, it was realized that more sensitive measurements other than the usual parameters of clinical assessment, BI, and the biopsy index have to be devised and developed. Better tools to measure directly and objectively the bactericidal activities of the various antileprosy drugs and their combinations are now necessary. Perhaps the use of the nude mouse is a beginning.
2. The percentage of patients with initial positive nose blows among the new untreated patients was much higher (60%-75%) than in relapsed patients (30%-40%). Positivity falls rapidly to 0% in 3 years in multidrug regimens such as those used in this study.
3. Clofazimine, even in low doses of 100 mg three times a week, was found to suppress the frequency and severity of ENL.
4. Toxicity was detected only in the regimen containing prothionamide combined with rifampin which produced a rise in SGPT in 40% of the patients. The rise, however, went back to normal when prothionamide was stopped.
5. Acceptability by the patients of the different regimens was generally good except for those regimens containing clofazimine which produced pigmentation. However, with proper motivation emphasizing the importance of the drug in the combination, the patient usually accepted it.
6. The drugs in the different regimens were practicable for use in the field except those containing clofazimine, which melts in tropical areas and the capsules tend to stick together. The addition of an inert powder to the container in which the clofazimine is stored solves this problem.
7. The prevalence of primary dapsone resistance in the three countries, determined by the mouse foot-pad test, was 22.4% in Thailand, 20.5% in Korea, and 6.1% in The Philippines.
8. The costs of the regimens were highest in those containing the most rifampin, as high as US$108.00 per patient for 5 years, and lowest in those containing only daily dapsone and a single dose rifampin, as low as US$57.75 per patient in 5 years. Given priority, the costs of the regimens are affordable in the three countries.
9. A multidrug regimen effective against both new and relapsed cases of lepromatous leprosy, whether dapsone sensitive or dapsone resistant, is recommended for field use.
10. The trials imparted valuable training to medical officers, laboratory technicians, and other ancillary personnel. This resulted in an upgrading of the general standard of leprosy control services in the three countries. Also, institutional laboratory facilities, including the mouse foot-pad test capability, were set up (where feasible) or improved, thereby enhancing and widening the services and research capabilities of these institutions. When the World Health Organization put forth its global multidrug therapy (MDT) standard regimen, the three countries of Thailand, The Philippines, and Korea had acquired basic experience from the JCT which proved valuable for instituting their national MDT programs.
Acknowledgments. We gratefully acknowledge with thanks the members of the Chemotherapy Experts Committee of Japan -Prof. Yoshiyasu Matsuo (Department of Bacteriology, Hiroshima University School of Medicine); Dr. Seitaro Okada (Director, National Sanatorium Oshima Seisho-en); and Dr. Shigenori Ishihara (Director, National Suruga Sanatorium)-for their technical support. We also wish to thank Drs. Roland Samson and Cesar J. Viardo for their advice in the conduct of the trial at Tala Leprosarium, Manila, The Philippines. Our profound gratefulness with thanks is also extended to the many physicians, nurses, medical technicians and attendants in the three participating countries for their varied contributions to the success of the trials whose names arc too numerous to mention individually here. Special mention has to be bestowed on Dr. Ricardo S. Guinto (now deceased) for his guidance in the planning and initial implementation of the trials. Our thanks also to Mr. Bernardo P. Mendoza, who did the computer work for the study, and to Mr. Fernando B. Arriola, for his clerical help in the preparation of this manuscript.
Funds for the study were provided by the Sasakawa Memorial Health Foundation of Tokyo, Japan.
REFERENCES
1. CARTEL, J. L., NAUDILLON, Y., ARTUS, J. C. and GROSSET, J. H. Hepatotoxicity of the daily combination of 5 mg/kg prothionamide + 10 mg/kg rifampin. Int. J. Lepr. 53(1985)15-18.
2. INTERNATIONAL SYMPOSIUM ON LEPROSY AND JOINT CHEMOTHERAPY TRIAL MEETING (Seoul and Anyang, Korea, May 30-June 2, 1978). Tokyo: Sasakawa Memorial Health Foundation, 1979.
3. JOINT CHEMOTHERAPY TRIALS STEERING COMMITTEE (JCT). Protocol and Manual. Cebu, The Philippines: Leonard Wood Memorial, 1979.
4. LEVY, L., SHEPARD, C. C. and FASAL, P. Clofazimine therapy of lepromatous leprosy caused by dapsone resistant Mycobacterium leprae. Am. J. Trop. Med. 21(1972)315-321.
5. LEVY, L., SHEPARD, C. C. and FASAL, P. Death of M. leprae following treatment of leprosy patients with 1500 mg. rifampin in a single dose. (Abstract) Int. J. Lepr. 41(1973)490.
6. LEVY, L. and RANDALL, H. P. A study of skin pigmentation by clofazimine. Int. J. Lepr. 39(1970)404-416.
7. PEARSON, J. M. H., CAP, J. A., HAILE, G. S. and REES, R. J. W. Dapsone resistant leprosy and its implications for leprosy control programmes. Lepr. Rev. 48(1977)83-94.
8. PEARSON, J. M. H., HAILE, G. S., BARNETSON, R. ST.C. and REES, R. J. W. Dapsone-resistant lep rosy in Ethiopia. Lepr. Rev. 50(1979)183-199.
9. PEDLEY, J. C. The nasal mucus in leprosy. Lepr. Rev. 44(1973)33-35.
10. PROCEEDINGS OF THE 1ST INTERNATIONAL WORKSHOP ON CHEMOTHERAPY OF LEPROSY IN ASIA (Manila, Philippines, January 26-28, 1977). Tokyo: Sasakawa Memorial Health Foundation, 1977.
11. QUAGLITO, R., BECHELLI, L. M. and MARQUEZ, R. M. Bacterial negativity and reactivation (relapse) of lepromatous outpatients under sulfone treatment. Int. J. Lepr. 38(1970)250-253.
12. RIDLEY, D. S. Histological classification and the immunological spectrum of leprosy. Bull. WHO 51(1974)451-165.
13. RIDLEY, D. S. and HILSON, G. R. F. A logarithmic index of bacilli in biopsies. Int. J. Lepr. 35(1967)184-186.
14. RODRIGUEZ, J. N. The histoid leproma (its characteristics and significance). Int. J. Lepr. 37(1969)1-21.
15. SHEPARD, C. C. Multiplication of M. leprae in the footpad of the mouse. Int. J. Lepr. 30(1962)291-306.
16. SHEPARD, C. C. Combinations involving dapsone, rifampin, clofazimine and ethionamide in the treatment of M. leprae infections in mice. Int. J. Lepr. 44(1976)135-139.
17. Standard protocol for trials of combined drug regimens among lepromatous patients. Report of the First meeting of the Thelep Scientific Working Group. TDR/SWG-THELEP (1)/77.3. Geneva, 25-29 April 1977.
18. WATERS, M. R. F. The diagnosis and management of dapsone-resistant leprosy. Lepr. Rev. 48 (1977) 95-105.
1. M.D.. D.P.H.(Lond.), Chief, Epidemiology Branch, Leonard Wood Memorial, Cebu, The Philippines.
2. M.D., M.P.H., Chief, Clinical Research Branch, Leonard Wood Memorial, Cebu, The Philippines.
3. M.D., Pathologist, Leonard Wood Memorial, Cebu, The Philippines.
4. D.V.M., Chief, Vivarium, Leonard Wood Memorial, Cebu, The Philippines.
5. M.D., Director, Institute for Leprosy Research, Korean Leprosy Control Association, Anyang, Korea.
6. M.D., Director, Department of Dermatology, Fatima Hospital, Daego, Korea.
7. M.D., Deputy-Permanent Secretary, Office of the Permanent Secretary, Ministry of Public Health, Bangkok, Thailand.
8. M.D., Director, Leprosy Division, Department of Communicable Disease Control, Ministry of Public Health, Soi Bamrasnaradoon, Muang District, Nonthaburi, Thailand.
9. M.D., Chief, Leprosy Control Service, Department of Health, Manila, The Philippines.
10. T. Ito, M.D., Ph.D., Professor Emeritus. Osaka University, Japan, now Senior Consultant. Raj-pracha-samasai Institute, Phrapradaeng District. Samutprakarn 10130, Thailand.
11. Y. Yuasa, M.D., Executive and Medical Director, Sasakawa Memorial Health Foundation, Tokyo. Japan.
Reprint requests to Dr. R. Cellona, Leonard Wood Memorial, c/o Cebu Skin Clinic, Cebu City 6000, The Philippines.
Received for publication on 27 April 1989.
Accepted for publication in revised form on 13 October 1989.