- Volume 58 , Number 1
- Page: 12–8
Effectiveness of pefloxacin in the treatment of lepromatous leprosy
ABSTRACT
As a first clinical trial of a fluoroquinolone derivative in leprosy, ten previously untreated lepromatous leprosy patients, about two fifths of them with primary dapsone resistance but all susceptible to rifampin, were treated with pefloxacin 400 mg twice daily for 6 months. Definite clinical improvement was observed in all ten patients as early as 2 months after beginning treatment, and the morphological index was also drastically decreased to the baseline during the same period. The rapid bactericidal effects, as measured by serial mouse footpad inoculations, were demonstrated to the extent that about 99% of the bacilli were killed during the first 2 months of treatment. However, the bacterial load, in terms ofthe bacterial index and the number of acidfast bacilli per mg of tissue, of the patients was only moderately reduced. The side effects were mild, and the patients tolerated the treatment well.
RÉSUMÉ
An cours d'un premier essai clinique des dérivés de la fluoroquinolone dans la lèpre, on a traité par la pefloxacine à raison de 400 mg deux fois par jour pendant 6 mois, dix malades atteints de lèpre lépromateuse et non traités antérieurement. Environ les deux cinquièmes de ces malades présentaient une résistance primaire à la dapsone; tous étaient néanmoins susceptibles à la rifampicine. Chez tous les malades, on a observé une amélioration clinique nette dans les deux mois ayant suivi le début du traitement. Au cours de la même période, l'index morphologique avait également diminué de manière dramatique rejoignant les valeurs de référence. La rapidité de l'effet bactéricide, mesurée par des inoculations en série du coussinet plantaire de la souris, a été démontrée, vu qu'environ 99% des bacilles étaient tués au cours des deux premiers mois de traitement. Toutefois, on n'a constaté qu'une réduction modérée de la charge bactérienne en terme d'index bactériologique et de nombre de bacilles acidorésistants par mg de tissu. Les effets secondaires étaient mineurs; les malades ont bien toléré le traitement.
RESUMEN
En un programa preliminar de tratamiento con un derivado de fluoroquinolona, 10 pacientes lepromatosos sin tratamiento previo, 4 de ellos con resistencia primaria a la dapsona pero todos susceptibles a la ritampina, se trataron con 400 mg de pefloxacina dos veces al dia durante 6 meses. En los 10 pacientes se observó una clara mejoría clínica a los 2 meses de iniciado el tratamiento y una drástica disminución en el indice morfológico. Los rápidos efectos bactericidas, medidos por inoculación seriada en la almohadilla plantar del ratón, mostraron que cerca del 99% de los bacilos fueron muertos durante los primeros dos meses del tratamiento. Sin embargo, la carga bacteriana, en términos de índice bacteriano, y el número de bacilos ácidoresistentes por mg de tejido, resultaron solo moderadamente reducidos. Los efectos colaterales fueron moderados y los pacientes toleraron bien el tratamiento.
Since dapsone-resistant leprosy has become a worldwide phenomenon (9), multidrug therapy (MDT) is a crucial approach for leprosy control programs as recommended by a World Health Organization (WHO) Study Group in 1981 (20). In view of the size of bacterial populations within leprosy patients, it was recommended that two drugs should be used simultaneously for the treatment of paucibacillary (PB) leprosy, for the treatment of multibacillary (MB) leprosy. According to the recommendations, only batericidal antileprosy drugs should be employed as components of MDT regimens. However, to date only four bactericidal antileprosy drugs, i.e., dapsone, rifampin, clofazimine, and a thioamide (ethionamide/prothionamide) are available for field use. Because dapsone is an effective, safe and cheap drug and rifampin displays extraordinarily strong bactericidal activity against Mycobacterium leprae (11,16), both drugs are the standard components of the MDT regimens for both PB as well as MB leprosy. As far as the thioamides are concerned, because they may cause hepatotoxicity especially when combined with rifampin, it was recommended by the recent WHO Expert Committee on Leprosy (19) that neither thioamide should be used as a component of MDT under field conditions except when absolutely necessary. Consequently, apart from the combination of dapsone, rifampin and clofazimine, the choice of an alternative MDT regimen for MB leprosy is practically nil. Therefore, the development of powerful and well-accepted new antileprosy drugs with bactericidal mechanisms entirely different from those of the existing drugs is urgently needed. It is also thought that the combination of powerful bactericidal drugs with rifampin may improve the efficacy of MDT and shorten the duration of treatment, with the hope that rifampin-resistant mutants may be eliminated more rapidly and completely by and three drugs the new drugs.
Fluoroquinolones, a new class of compounds characterized by a broad antimicrobial spectrum including mycobacteria (,2, 4, 18) together with limited toxicity (7, 17), have recently been introduced into chemotherapy for various human infectious diseases (1, 7, 17). Pefloxacin, one of the members of this class, was recently demonstrated to be bactericidal against M. leprae in the mouse foot-pad model (6). This represents the first lead to an important new antileprosy drug in many years. In order to confirm the bactericidal activity of pefloxacin in human leprosy, and also to evaluate its potential side effects, a 6-month clinical trial of the drug was conducted among previously untreated lepromatous patients in Adzope, Cote d'lvoire.
MATERIALS AND METHODS
Patients. Ten previously untreated lepromatous leprosy patients were selected for the trial and were hospitalized at the Institut Raoul Follereau, Adzope, Cote d'lvoire, between July 1985 and April 1986. To ensure that the patients had not received antileprosy treatment in the past, during the course of selection a clinical history and a urine analysis (8) were undertaken on all candidates at their first visit to the clinic. Among the 10 patients selected, 7 were classified as polar lepromatous (LL) and 3 as borderline lepromatous (BL); 7 were males and 3 were females aged between 24 and 39 (31.9 ± 3.5). All of them exhibited active skin lesions with a high bacterial index (BI) and morphological index (MI) (Table 2). None of them had a recent history of erythema nodosum leprosum (ENL).
Initial examination. After admission, the following examinations were carried out on each patient: a thorough physical examination, especially the examination for leprosy, photographs of the skin lesions; a lepromin test; a PA chest X-ray; skin smears (for measurement of BI, and the smears were taken from six sites including both ear lobes and four other sites selected by the clinical investigator); blood and urine analyses; measurement of serum glutamic-pyruvic transaminase activity and serum bilirubin level; and a skin biopsy. All these examinations followed the THELEP standard protocol (13). The first, or pretreatment, biopsy was taken from an active lesion large enough to provide several specimens. The biopsy specimen was divided into two; one portion was fixed in formol saline for histopathological examination, the other portion was shipped in a refrigerated container (NUNC, Roskilde, Denmark) by air freight to the Faculté de Médecine Pitié-Salpêtrière, Paris, for mouse foot-pad inoculation. The intervals between the biopsy taken in Adzopé and the completion of mouse inoculation in Paris ranged from 48 to 72 hr. During processing of the specimen for inoculation, the MI and the counting of acidfast bacilli (AFB) per mg of tissue were carried out.
For mouse inoculation, 4-week-old, immunologically intact (normal), female Swiss mice (purchased from the Janvier Breeding Centre, 53680 le Genest, France) were used. All mice were inoculated according to Shepard's method (14). There were two different purposes for mouse inoculation: one was to measure the proportion of viable organisms in the bacterial populations before treatment; the other was to measure the susceptibility status of the pretreatment organisms to dapsone and rifampin.
Depending upon the purpose of the mouse foot-pad inoculation, the number of organisms inoculated per foot pad and the dates of harvest were varied. To measure the proportion of viable M. leprae, serially diluted suspensions were prepared from each specimen, and groups of 10 mice were inoculated with 5 X 103, 5 X 102 and 5 X 101AFB per foot pad. Fourteen months later, the mice were sacrificed and the organisms were harvested from individual inoculated foot pads. M. leprae were considered to have multiplied when at least 105 AFB per foot pad were harvested. The proportion of viable organisms was calculated through the analysis of the median infectious dose (ID50) (15). To measure the susceptibility of the organisms to dapsone and rifampin, standard procedures (10) were followed. In brief, an additional 40 mice were inoculated in their left hind foot pads with organisms recovered from each pretreatment biopsy. The inoculum size was 5 X 103 AFB per foot pad and the mice were divided into five groups of equal number. One group was maintained as the untreated control group and the remaining groups were treated either with dapsone incorporated into the diet at a concentration of 0.0001 or 0.001 or 0.01 g per 100 g diet, or with rifampin in a weekly dose of 10 mg per kg body weight, by esophageal canula. Harvests of M. leprae from the inoculated foot pads of three untreated mice were performed approximately 8 months after inoculation, and repeated at intervals of 2 months if necessary, until a significant and universal multiplication of M. leprae was detected, i.e., the number of organisms was found to be at least 105 in most harvested foot pads. At this time, harvests were performed from additional untreated mice and treated mice as well. If multiplication of M. leprae, with the same criterion as described already, was detected in at least one treated mouse, drug resistance was diagnosed. For dapsone, the degree of resistance was defined as low, intermediate or high, depending on the ability of the organisms to multiply in mice administered dapsone in a concentration of, respectively, 0.0001, 0.001 or 0.01 g per 100 g diet.
Pefloxacin treatment. After completion of the above-mentioned examinations, pefloxacin monotherapy was started. The drug was obtained from Roger Bellon Laboratories (Paris, France) as a gift, and was given under supervision 400 mg twice daily, with an interval of 12 hr between each administration. After completion of the 6-month trial, all the patients were treated by the WHO/MDT regimen for MB leprosy with a minimal duration of 2 years.
Examination during treatment. To evaluate the response to the treatment and the side effects, most of the examinations mentioned previously were repeated regularly. Every month, apart from repeating the blood and urine analyses and liver function tests, a clinical examination was carried out, and the patients were interviewed for symptoms suggesting adverse reactions to the drug. The occurrence of leprosy reactions, ENL and reversal reaction was carefully monitored. Skin smears, from the same sites as the initial examination, were repeated every 3 months. Skin biopsies were repeated, from the same lesion, every 2 months and shipped to Paris for the measurement of the MI, number of AFB per mg of tissue, and the proportion of viable organisms through mouse foot-pad inoculation.
RESULTS
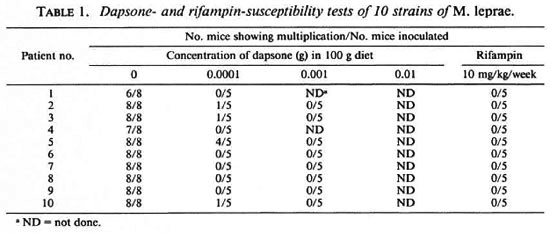
Drug susceptibility of the stains. The results of drug susceptibility testing of the organisms from the pretreatmcnt specimens are given in Table 1. According to the definition of multiplication, all of the strains multiplied in the control mice, but four of them (case nos. 2, 3, 5 and 10) showed multiplication in mice administered dapsone at a concentration of 0.0001 g per 100 g diet. None of these strains were able to multiply in the foot pads of mice fed the 0.001 g per 100 g dapsone-incorporated diet. Because of this, no harvest was performed from the foot pads of mice receiving dapsone in the highest concentration, i.e., 0.01 g per 100 g diet. In addition, all 10 strains were unable to multiply in mice treated with 10 mg/kg rifampin once weekly. In brief, 4 out of the 10 strains showed low-degree primary dapsone-resistancec but all of them were rifampin sensitive.
Clinical response to pefloxacin treatment. The clinical response during the trial period was very impressive. In fact, definite clinical improvement, manifested as regression of the infiltration, flattening of the nodules and amelioration of nasal obstruction, was noticed in all 10 patients as early as 2 months after the beginning of treatment. The improvement continued until the end of the trial. Some lesions, mainly small nodules, disappeared in the course of the 6 months of treatment. As in the case of treatment with other effective antileprosy drugs, marked improvement was observed more often among those patients who had more severe pretreatment lesions, such as nodules, plaques and histoid lepromas, apparently because the improvement of such lesions was easier to discern.
Side effects. Pefloxacin appeared to be well tolerated throughout the 6-month treatment. Neither cutaneous photosensitivity nor neuropsychic disorders were observed. Two patients complained of abdominal pain, one of joint pain, another of pruritis and two of dizziness. Although it is uncertain whether or not these were side effects due to pefloxacin treatment, all of the complaints were mild and none of them led to withdrawal of the treatment. The laboratory findings also showed good tolerance with all parameters, including the monthly blood and urine analyses and liver function tests, remaining within normal ranges throughout the trial.
Leprosy reaction. Neither ENL nor reversal reaction was observed during the trial.
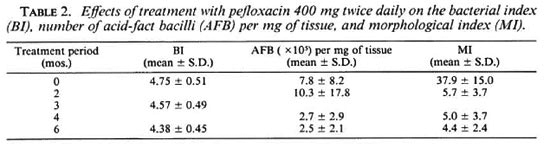
Bacteriological response. Before treatment, as shown in Table 2, the bacterial load, in terms of BI and number of AFB per mg of tissue, and the MI of all these patients were high. As expected, after the 6-month pefloxacin treatment, the average BI of the group was reduced by 0.37 log, i.e., from 4.75 ± 0.51 (mean ± S.D.) before treatment to 4.38 ± 0.45 at the end of the trial. The same level of reduction was also observed by the actual counting of AFB per mg of tissue. It was (7.8 ± 8.2) X 105 before treatment and (2.5 ± 2.1) X 105 6 months later. However, the fall in the MI was more rapid. The first measurement of the MI during treatment was performed 2 months after the beginning of the therapy when it had already dropped drastically to 5.7 ± 3.7% (p < 0.01), very close to the baseline according to the criterion used in our laboratory. Afterwards, the MI remained at the same level until the end of the trial.
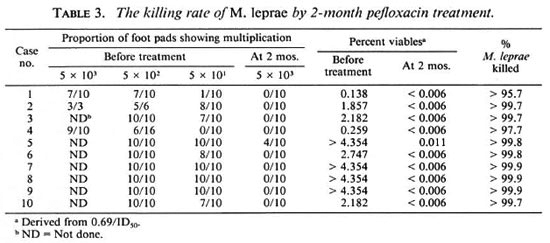
Killing of M. leprae by pefloxacin. The killing rate of M. leprae, demonstrated through foot-pad inoculation with M. leprae recovered from serial biopsies, taken before and during treatment with pefloxacin, are presented in Table 3. Viable organisms were detected from all pretreatment biopsies, but the proportion of viables varied from 0.14% to more than 4.35%. Two months after the beginning of treatment, 9 out of 10 strains lost their infectivity to mice which received the highest inocula, i.e., 5 X 103 per foot pad; the only strain which showed multiplication was recovered from patient no. 5, and the multiplication was only observed in 4 out of 10 mice receiving the highest inocula but not in those receiving the lower inocula, i.e., 5 x 102 and 5 x 101 per foot pad. The proportion of viable organisms at 2 months ranged from <0.006% to 0.011%. Therefore, about 99% of viable organisms were killed during the first 2 months of treatment with pefloxacin. The next biopsy was taken at 4 months, and none of the strains showed multiplication in mice (data not shown). Apparently by that time all of the strains had lost their infectivity to mice, even those receiving the highest inocula. Therefore, it was decided not to harvest the mice inoculated with bacilli recovered from biopsies taken at 6 months.
DISCUSSION
As a byproduct of the clinical trial of pefloxacin, the purpose of the drug susceptibility tests in the trial was to measure the level of primary dapsone resistance and the occurrence of primary rifampin resistance in Côte d'Ivoire, because these two compounds are the most important components of the WHO/MDT regimens for both MB and PB leprosy. The results indicated that the frequency of primary dapsone-resistant leprosy in Côte d'Ivoire, about two fifths of previously untreated MB patients, was similar to other leprosy-endemic countries, and the resistance was of a low degree (5,9). Similar to the results obtained from other parts of the world (5), no primary rifampin resistance has been detected among this group. As expected, the clinical and bacteriological responses of these dapsone-resistant patients to pefloxacin were exactly the same as those of dapsone-susceptible patients.
In our earlier report (6), because pefloxacin showed strong bactericidal activity against M. leprae in the mouse foot-pad system, and also because the pharmacokinetics of pefloxacin are more favorable in man than in the mouse (12), we suspected that pefloxacin would display bactericidal activity against M. leprae in human leprosy. The results of the current clinical trial clearly confirm this anticipation. Although it is well known that the assessment of clinical improvement in leprosy is very subjective and difficult to quantify, definite clinical improvement was observed in all 10 lepromatous patients as early as 2 months after commencing pefloxacin treatment. The MI, an indirect but less-sensitive indicator of the viability of M. leprae, also decreased drastically during the same period. More important is the direct demonstration of the rapid bactericidal effects as measured by the serial mouse foot-pad inoculations. Nine of the 10 strains lost their infectivity to mice, and about 99% of the bacilli were killed during the first 2-month period of treatment; none of the strains were able to infect mice 4 months after the beginning of the treatment. Except for rifampin (11, 16), no other drugs thus far tested have demonstrated such a degree of bactericidal activity. In fact, the 2-month biopsy was the first one taken during the treatment; it is possible that a significant number of M. leprae might have been found to have been killed earlier than 2 months if the first biopsy had been taken earlier. The moderate reductions in the BI and the number of AFB per mg of tissue during pefloxacin treatment were expected. This is because both parameters measured the total bacterial load including both dead and viable organisms, because the majority of bacilli were dead in lepromatous patients, even before treatment as shown in the current study, and also because the dead organisms persisted in the tissue of lepromatous patients and were only slowly eliminated. Both parameters only decreased by about an annual rate of 0.7 to 0.9 log10 units, which is very similar to the results obtained from other trials with effective regimens, despite a very rapid killing.
As for any antimicrobial agent, usually the duration of treatment with fluoroquinolones is relatively short, from several days to a few weeks. Although fluoroquinolones have proven to be a safe group of antibiotics, very little information relative to the side effects of long-term fluoroquinolone treatment is available (17). In the present study, the side effects were mild during 6 months of pefloxacin treatment. Nevertheless, the sample size was small. The possible side effects (7, 17), such as serious gastrointestinal symptoms, cutaneous photosensitivity, and disorders of central nervous system, should be borne in mind in future fluoroquinolone trials in leprosy. In addition, until the problem of possible inhibition of cartilage growth during fluoroquinolone treatment has been clarified in humans, the use of pefloxacin, and other fluoroquinolones, should be avoided in children and in pregnant women.
It was recently discovered that ofloxacin, another fluoroquinolone, administered to mice in a kinetic experiment in a daily dosage of 50 mg per kg displayed bactericidal activity similar to that of pefloxacin administered in a daily dosage of 150 mg per kg, and that ofloxacin in a daily dosage of 150 mgper kgwas fully bactericidal (3). The estimated minimum inhibitory concentration (MIC), about 4 µg per ml, can be obtained and maintained in patients by administration of ofloxacin in a welltolerated dosage, such as 400 mg daily. Because of this, and also as a continuation of the clinical trial ofpefloxacin, a controlled clinical trial is being conducted. This trial was designed to compare more precisely the bactericidal activities of pefloxacin and ofloxacin in human leprosy, and to evaluate the side effects of pefloxacin/ofloxacin in combination with the WHO/MDT regimen for MB leprosy. The next steps in clinical trials should be studies of the therapeutic effects of combined regimens containing pefloxacin/ofloxacin and other existing antileprosy drugs, e.g., dapsone, clofazimine and rifampin, for MB leprosy, and should try to identify the optimal dosages, rhythms and durations of pefloxacin/ofloxacin in combination with other antileprosy drugs.
Acknowledgment. This investigation received financial support from the UNDP/World bank/WHO Special Programme for Research and Training in Tropical Diseases. We thank Corinne Beoletto for her technical assistance and Roger Bellon Laboratories, Paris, for supplying us with pefloxacin.
REFERENCES
1. ESPLACES, N., GUTMANN, L., CARLET, J., Gui¬ BERT. J. and ACAR, J. F. The new quinolones and their combinations with other agents for therapy of severe infections. J. Antimicrob. Chemother. 17Suppl.A(1986)25-39.
2. GARCÍARODRÍGUEZ,J.A.Activityofquinoloncs against mycobacteria in vitro and in vivo. Quinoloncs Bull. 4 (1988)21-25.
3. GROSSET, J. H., GUELPALAURAS, C. C, PERANI, E. G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
4. GROSSET, J. H., JARLIER, V., TRUFFORT-PERNOT, C, GUELPA-LAURAS, C. C. and LECOEUR, H. Activité des nouvelles quinolones sur les mycobactéries. In: Les Nouvelles Quinolones. Pocidalo, J. J., Vachon, F. and Régnier, B., eds. Paris: Arnette, 1985, pp. 181-189.
5. GUELPA-LAURAS, C. C, CARTEL, J. L., CONSTANT-DESPORTES, M., MILLAN, J., BOBIN, P., GUIDI, C, BRUCKER, G., FLAGEUL, B., GUILLAUME, J. C, PICHET, C, REMY, J. C. and GROSSET, J. H. Primary and secondary dapsone resistance of M. leprae in Martinique, Guadeloupe, New Caledonia, Tahiti, Senegal, and Paris between 1980 and 1985. Int. J. Lepr. 55(1987)672-679.
6. GUELPA-LAURAS, C. C, PERANI, E. G., GIROIR, A. M., and GROSSET, J. H. Activities of pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
7. HOOPER, D. C. and WOLFSON, J. S. The fluoroquinolones: pharmacology, clinical use and toxicities in humans. Antimicrob. Agents Chemother. 28(1985)716-721.
8. HUIKESHOVEN, H. and MADARANG, M. G. Spot test for detection of dapsone in urine: an assessment of its validity and interpretation in monitoring dapsone self-administration. Int. J. Lepr. 54(1986)21-24.
9. JI, B. H. Drug resistance in leprosy; a review. Lepr. Rev. 56(1985)265-278.
10. JI, B. H. Drug susceptibility testing of Mycobacterium leprae. Int. J. Lepr. 55 (1987) 830-835.
11. LEVY, L., SHEPARD. C. C. and FASAL, P. The bactericidal effect of rifampicin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
12. MONTAY, G., GOUEFFON, Y. and ROQUET, F. Absorption, distribution, metabolic rate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob. Agents Chemother. 25(1984)463-472.
13. REPORT OF THE FIRST MEETING OF THELEP SCIENTIFIC WORKSHOP GROUP. Annex I. Standard protocol for chemotherapy trials in lepromatous leprosy. TDR/SWG-THELEP(1)/77.3.
14. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112(1960)445-454.
15. SHEPARD, C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae. Int. J. Lepr. 50(1982)96-101.
16. SHEPARD, C. C, LEVY, L. and FASAL, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1124.
17. SMITH, C. R. The adverse effects of fluoroquinolones. J. Antimicrob. Chemother. 19(1987)709-711.
18. WOLFSON, J. S. and HOOPER, D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra ofactivity in vitro. Antimicrob. Agents Chemother. 28(1985)581-586
19. WHO EXPERT COMMITTEE ON LEPROSY. Sixth Report. Geneva: World Health Organization Tech. Rep. Ser. 768.
20. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D., Institut Raoul Follereau, Adzopé, Côte d'Ivoire.
2. M.D., Faculté de Médecine Pitié-Salpêtrière, 91 Boulevard de l'Hôpital, 75634 Paris Cedex 13, France.
3. E. G. Perani, Technician, Faculté de Médecine Pitié-Salpêtrière, 91 Boulevard de l'Hôpital, 75634 Paris Cedex 13, France.
4. M. D., Faculté de Médecine Pitié-Salpêtrière, 91 Boulevard de l'Hôpital, 75634 Paris Cedex 13, France.
Reprint requests to Dr. J. H. Grosset.
Received for publication on 21 June 1989.
Accepted for publication in revised form on 29 August 1989.