- Volume 58 , Number 1
- Page: 19–24
Relapses in paucibacillary leprosy after MDT - a clinical study
ABSTRACT
A study was undertaken in a field-based project to assess the incidence and clinical profile of relapses occurring in paucibacillary leprosy patients after adequate doses of multidrug therapy (MDT). Of the 1509 paucibacillary patients who had received not less than 6 doses of MDT (WHO regimen), 85 relapsed; a relapse rate of 5.63% (17.5/1000 person years at risk). These relapses included 12 cases with features of reversal reaction. Seventy-nine percent of the patients relapsed with skin lesions. The relapse rate was higher in borderline cases and in those with more lesions, and it was lower in those who had received dapsone for at least 6 months after cessation of MDT. Seventy-four percent of the relapses were detected between 7 and 24 months of follow up. We feel that uniform clinical criteria should be formulated for the diagnosis of relapse. Individualization of therapy, rather than adhering to a fixed duration of MDT, would be likely to achieve satisfactory cure rates and fewer relapses.
RÉSUMÉ
Dans un projet sur le terrain, on a entrepris une étude afin d'évaluer l'incidence et le profil clinique des récidives qui surviennent chez des malades atteints de lèpre paucibacillaire, après un traitement par des doses adéquates de polychimiothérapie (MDT). Parmi les 1509 malades paucibacillaires qui avaient reçu 6 doses ou davantage de MDT (posologie de l'OMS), 85 ont présenté des récidives. Le taux de récidive a été de 5,63% (17,5 pour mille personne/années à risque). Ces récidives comprenaient douze cas qui témoignaient des caractéristiques d'une réaction réverse. Soixante dixneuf pour cent des malades ont présenté des lésions cutanées lors de la récidive. Le taux de récidive a été plus élevé que dans les cas dimorphes et que chez les malades qui présentaient davantage de lésions. Ce taux était plus faible chez les patients ayant reçu de la dapsone pour au moins 6 mois après l'interruption de la polychimiothérapie. Soixante quatorze pour cent des récidives ont été détectées entre 7 et 24 mois. On estime que des critères cliniques uniformes devraient être établis pour diagnostiquer les récidives. Plutôt que de s'en tenir à une durée fixe de polychimiothérapie, il faudrait individualiser la thérapeutique, car on obtiendrait vraisemblablement ainsi des taux de guérison satisfaisants, avec moins de récidives.
RESUMEN
Se realizó un estudio de campo para establecer la incidencia de recaídas y su perfil clínico, en pacientes con lepra paucibacilar después de administrar una terapia adecuada con múltiples drogas (TMD). De los 1509 pacientes paucibacilares que habían recibido no menos de 6 dósis con múltiples drogas (MD) (esquema de la OMS), 85 recayeron; una incidencia de recaída del 5.63% (17.5/1000 personas en riesgo). Estas recaídas incluyeron 12 casos con las características de una reacción reversa. El 79% de los pacientes recayeron con lesiones en la piel. La incidencia de recaídas fue mayor en los casos limítrofes y en aquellos con más lesiones, y fue menor en aquellos que habían recibido dapsona cuando menos 6 meses después de terminar la TMD. El 74% de las recaídas se detectó entre 7 y 24 meses de seguimiento. Pensamos que deben formularse criterios clínicos uniformes para el diagnóstico de las recaídas. La terapia individualizada más que el tratamiento general con MD por un tiempo preestablecido, aseguraría mejores niveles de curación y menos recaídas.
The two most important criteria for the evaluation of a treatment regimen in any infectious disease are: a) the amelioration of symptoms and signs, and b) the incidence of relapse (16).
More than 6 years have elapsed since the worldwide introduction of the World Health Organization (WHO) recommended multidrug therapy (MDT) for both paucibacillary and multibacillary forms of leprosy (28). Its efficacy is well recognized; however, very few studies have been undertaken to assess relapses following MDT, especially under field conditions (18, 20, 21). We felt, therefore, that such a study in paucibacillary leprosy would be relevant.
MATERIALS AND METHODS
This study was carried out in a field-based leprosy control unit covering two large municipal wards of Greater Bombay, India, with a population of over one million. Patients with a negative skin smear before starting therapy (including pure neuritic cases) were included. The cases were classified broadly according to the classification adopted by the Indian Association of Leprologists (8). However, in the case of a single lesion, the suggestion of Job and Chacko was followed, in that if a single patch had satellite lesions, it was classified as borderline leprosy (11). MDT was administered according to the WHO guidelines for paucibacillary (PB) leprosy with a few modifications: a) MDT was given to the point of clinical inactivity (7, 19) and not arbitrarily stopped at six doses. Inactivity was defined as the absence of the signs of activity which are: i) thickening or erythema of skin lesions, ii) change in size or number of skin lesions, iii) thickening and tenderness of nerves (4). If the patient was still active at 12 doses, clofazimine was usually added as per the schedule recommended by WHO for multibacillary (MB) leprosy. The discretion for starting clofazimine at this stage was left to the responsible physician. However, if the patient was still active at 18 doses, then clofazimine was given in all cases and the triple MDT was continued to the point of inactivity, b) Patients with more than five lesions were usually given clofazimine from the outset.
Compliance was checked by random tablet counts and urine tile testing for dapsone.
In all cases, inactivity was decided on clinical grounds alone, which are known to be rather subjective and ill-defined. In patients in whom, after adequate doses of rifampin, the achievement of inactivity was uncertain, dapsone (DDS) was continued for a minimum period of 6 months or until we were reasonably assured of clinical inactivity. Eventually, 57% of our patients were given post-MDT dapsone.
Patients were followed up, either at the clinics or at their homes, by doctors or paramedical workers at 6-month intervals. They were also asked to report immediately if they observed signs of relapse (which were explained to them at the time of release from treatment). We have included in this study only those cases in whom at least one 6-month post-therapy assessment was made. Of the 2219 PB cases who had completed their schedule of treatment, 1509 fulfilled this criterion and are reported here.
The criteria of relapse were predominantly clinical (12,15): a) extension, thickening, erythema or infiltration of existing lesions or the appearance of new lesions suggestive of leprosy; b) thickened tender nerves and new paralysis of muscles, and c) bacteriological positivity. However, we did not have any patient relapsing with MB leprosy during the study period.
Histology was done in only 10 cases. In each ofthese cases, a 58 mm punch biopsy was taken from the suspect lesion and subjected to hematoxylin and eosin and Fite-Faraco stains.
In all cases of relapse, MDT was recommended with three drugsdapsone, rifampin, and clofazimine.
The surveillance period ranged from 6 months to 5 years, and the total number of person years at risk (PYR) was 4842. Lepromin testing was done at random in 874 patients at the commencement of treatment. Skin smears were repeated at yearly intervals.
RESULTS
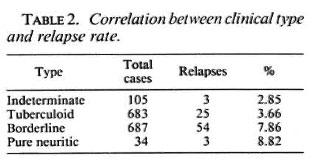
We diagnosed 85 relapses with a relapse rate (RR) of 5.63% (17.5/1000 PYR): 79% of the patients relapsed with skin lesions, 15% had neuritis, and 6% had a mixed pattern. Ofthose who relapsed in the skin, 63% manifested reactivation in the original lesions, 31% had new lesions, while the rest had both. Of the nerve relapses, 67% had recurrence in the original nerves, 22% had new nerve involvement, and the remaining had both (Table 1).
Twelve patients, all borderline (B) type leprosy, had lesions suggestive oftype 1 reaction (erythema, swelling, scaling) and could well have been cases of the socalled late reversal reaction (27).
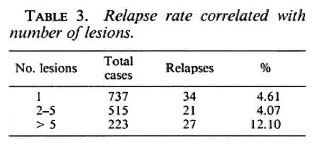
The RR was significantly higher in the borderline compared to the tuberculoid group (p < 0.01) (Table 2). The RR in patients with multiple lesions was significantly higher than in those with 15 lesions (χ2= 16.03 with Yates' correction; p < 0.001; DF = 1) (Table 3).
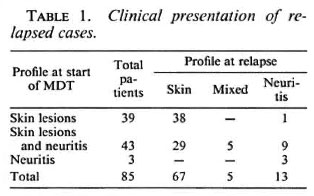
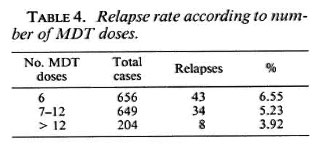
Our data did not indicate any significant correlation in the RR with cither regularity oftreatment or lepromin status. Among the patients given six doses, the RR was 6.55%; the corresponding figures for those given 7-12 doses and more were 5.23% and 3.92%, respectively. Although there was a trend for higher relapse rates in those given shorter MDT courses, this was not statistically significant (Table 4). This trend was also apparent when relapse rates were correlated with the type of regimen and number of doses given (Table 5). For this purpose, patients who were started on clofazimine at 12 or 18 doses were considered to be in the threedrug group.
We analyzed the borderline cases to see if the RR correlated with nerve involvement and the type of therapy given. Generally, there was a trend of higher RR with increasing nerve involvement, although it was not statistically significant. Also, the threedrug regimen seemed to reduce the RR, even when nerve involvement was more extensive (Table 6). For this purpose also, those patients in whom clofazimine was started at 12/18 doses were considered to be in the threedrug group.
The RR was significantly reduced in those patients in whom dapsone was continued beyond cessation of MDT (p <0.01) (Table 7).

Seventy-four percent ofour relapses were detected between 7and 24 months after cessation of therapy, 12% were detected within 6 months, and the rest after 2 years. This was statistically significant (p < 0.01).
The histopathology in 10 cases can be summarized as follows: Epidermal changes, comprising atrophy with short distorted rete ridges indicating the latter's regeneration, were observed in eight slides. Vacuolar degeneration of basal cells was seen in two slides, indicating their participation in active disease. Dermal changes were documented by the scarring of the papillary and reticular pars with paucity of nerves and adnexae in six cases. The capillaries and venules were dilated with endothelial cell proliferation. A mononuclear infiltrate containing many plasma cells interspersed with gross edema surrounded the vessels. Granuloma formation in the reticular dermis was observed in six cases. The histiocytes were dominantly noncommitted but occasional. Epithelioid cells, vacuolar and mulberry-like cells were noted. Bulky granulomas (22) were seen in two sections.
Seven of the ten slides could be labeled as relapses by the occurrence of granulomas, vascular changes, and inflammatory phenomena in a scarred dermis. The epidermal changes described above and the scarred dermis suggested a healed milieu. Superimposition of inflammatory pathology suggested recurrence, and the large number of plasma cells indicated an altered immunity. All of the slides could be placed in the BT-BL leprosy spectrum.
DISCUSSION
An assessment of the relapse rate (RR) provides a good operational indicator of the ultimate value of any therapeutic regimen in leprosy (29). Ideally, the aim of any treatment is to have a zero RR; in practice, especially under field conditions, this may not be possible. Our overall RR of 5.63% is comparable to that reported by others who had relapse rates of 2.9% (24), 4% (21), 5.9% (20), 12% (18), and 13.9% (13). Other workers have reported substantially lower rates of 0.28% (6), 0.42% (1), and 0.7% (10). Even a zero RR has been reported (3). We feel these discrepancies could largely be due to the different criteria for the diagnosis of relapse (e.g., some exclude the late reversal reaction from its scope) and the differing periods of follow up.
The cumulative years of follow up is also important. Our RR is 1.75/100 PYR. Pattyn, et al. (17) reported an overall RR of 2.84/100 PYR with various combinations of dapsone and rifampin. When clinical relapses were considered alone, the rate was halved. We find that these RR are higher than those reported with monotherapy (5, 9, 14). As more studies are undertaken, the picture may become clearer. It is felt that standardized criteria should be formulated for diagnosing relapses, especially under field conditions.
We found a higher RR in the borderline leprosy group compared to the other groups. Similar findings have been reported with both MDT (20) and with monotherapy (9, 16, 23)
It is stated that the differentiation of relapse from the late reversal reaction is difficult (26, 27), although some attempt to do so (1). We feel that such a distinction is not meaningful under field conditions, especially since reactions and neuritis can be manifestations of relapse (7, 21, 23, 30). Hence, we did not attempt to differentiate between the two except to note the fact that 12 cases had a reaction-like presentation.
The histological picture is not the ideal criterion for diagnosing relapse since it does not reflect the bacteriological event. Furthermore, it is difficult if not impossible to distinguish relapses from late reversal reactions, both on clinical grounds and by histopathology (17). We have a similar view and, hence, have not attempted to differentiate these two phenomena. We also feel that due to operational constraints, relapses will have to be diagnosed and managed on clinical grounds alone in the vast majority of patients.
The RR increased with increasing number of lesions, as reported by others (9).
Contrary to Jesudasan, et al.'s findings (9), we had a similar RR in both lepromin-positive and lepromin-negative individuals. Katoch, et al. (12) reported that 80% of the patients in whom treatment had to be restarted were lepromin positive. Regularity in treatment also did not make much difference in our series, similar to the findings of Pandian, et al. (15) and Rangaraj and Rangaraj (20). However, other workers (9) have different opinions.
Eighty-seven percent of our patients who relapsed did so within the first 2 years of discontinuation of therapy; around 75% were detected between 7 and 24 months of follow up. Similar data have been reported by others (2, 5, 16). Thus, a minimum surveillance period of 2 years is mandatory.
Does the administration of MDT lower the relapse rate? It is probably too early to draw a conclusion. Unlike others (25), we feel that it does not. However, we did note that in patients in whom dapsone was continued after clinical inactivity was achieved and MDT stopped, there was a lower incidence of relapse. Katoch, et al. (13) reported similar findings. This could possibly be explained by the fact that it is often very difficult to decide, under field conditions, if the patient is definitely inactive, and therefore the point at which therapy should be stopped is bound to be subjective (16). Some patients may be discharged when still active, and in these patients the chances of relapse would be higher. In this context, the WHO recommendation for giving only six doses of MDT in all PB patients (irrespective of the number of lesions, nerve involvement, etc.) needs further investigation.
Individualization of treatment would be likely to achieve satisfactory cure rates and acceptably low relapse rates.
REFERENCES
1. BOERRIGTER, G., PONNIGHAUS, J. M. and FINE, P. E. M. Preliminary appraisal of a WHO-recommended multiple drug régimen in paucibacillar leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-117.
2. BOURLAND, J., JANSSENS, L., GROENEN, G., PATTYN, S. R. and THE COLLABORATIVE STUDV GROUP ON THE TREATMENT OF LEPROSY. Incubation time of relapses after treatment of paucibacillary leprosy. (Abstract) Int. J. Lepr. 52(1984)686.
3. DAY, R. S. and TETERISSA, M. R. Field trial of short course combined chemotherapy in paucibacillary leprosy. (Abstract) Int. J. Lepr. 57 Suppl.(1989)340.
6. DHARMENDRA. Sulphone therapy. In: Leprosy, Volume I. Dharmendra, ed. Bombay: Kothari Medical Publishing House, 1978, pp. 359-412.
5. EKAMBARAM, V. Duration of treatment for disease arrest of non-lepromatous cases and relapse rates in these patients. Lepr. Rev. 50(1979)297-302.
6. GANAPATI, R. Efficacy of chemotherapy in paucibacillary leprosy; epidemiological issues and assessment problems. In: Indo-U.K. Workshop on Leprosy Research. Agra: Central JALMA Institute for Leprosy (ICMR), 1989.
7. GIRDHAR, B. K. Multidrug therapy in leprosy. Indian J. Lepr. 59(1987)145-151.
8. INDIAN ASSOCIATION OF LEPROLOGISTS. Clinical, histopathological and immunological features of the five-type classification approved by the Indian Association of Leprologists. Lepr. India 54(1982)22-25.
9. JESUDASAN, K., CHRISTIAN, M. and BRADLEY, D. Relapse rate among nonlepromatous patients released from control. Int. J. Lepr. 52(1984)304-310.
10. JESUDASAN, K., PANNIKAR, V. K., MANI MOZHI, N. and CHRISTIAN, M. Relapse rates in paucibacillary leprosy-WHO regimen versus dapsone monotherapy. (Abstract) Int. J. Lepr. 57 Suppl.(1989)340.
11. JOB, C. K. and CHACKO, C. J. G. A simplified 6 group classification of leprosy. Lepr. India 54(1982)26-32.
12. KATOCH, K., RAMU, G. and RAMANATHAN, U. Short term WHO advised multiple drug treatment of paucibacillary patients. (Letter) Indian J. Lepr. 58(1986)351-353.
13. KATOCH, K., RAMU, G. and RAMANATHAN, U. Evaluation of three short term regimens containing rifampin for the treatment of paucibacillary leprosy. (Abstract) Int. J. Lepr. 57 Suppl.(1989)340.
14. PANDYAN, T. D., SEETHAMBARAM, M., BHARATHI, R. and RAMU, G. A study of relapse in nonlepromatous and intermediate forms of leprosy in the ELEP leprosy control project, Dharampuri. (Abstract) Int. J. Lepr. 52 Suppl.(1984)686.
15. PANDYAN, T. D., SEETHAMBARAM, M., BHARATHI, R. and RAMU, G. A study of relapse in nonlepromatous and intermediate group of leprosy. Indian J. Lepr. 57(1985)149-158.
16. PATTYN, S. R. Incubation time for relapses after treatment of paucibacillary leprosy. Lepr. Rev. 55(1984)115-120.
17. PATTYN, S. R., GROENEN, G., BOURLAND, J., GRILLONE, S., JANSSENS, L. and THE COLLABORATIVE STUDY GROUP FOR THE TREATMENT OF LEPROSY IN ZAIRE AND RWANDA. A controlled therapeutic trial in paucibacillary leprosy com paring a single dose of rifampicin followed by 1 year of daily dapsone with 10 weekly doses of rifampicin. Lepr. Rev. 58(1987)349-358.
18. PAVITHRAN, K. Relapse of paucibacillary leprosy after short course of multidrug therapy. Indian J. Lepr. 60(1988)225-229.
19. RAMU, G. Multidrug therapy of leprosy. Indian J. Lepr. 57(1985)465-477.
20. RANGARAJ, M. and RANGARAJ, J. Experience with multidrug therapy in Sierra Leone: clinical, operational and managerial analysis. Lepr. Rev. 57 Suppl.3(1985)77-91.
21. REDDY, K. P. and MOHINUDDIN, S. K. Pattern of relapses in paucibacillary leprosy patients treated with MDT (WHO 1982). Indian J. Lepr. 60(1988)581-588.
22. RIDLEY, D. S. Skin Biopsy in Leprosy. 2nd ed. Basle: CIBA-GEIGY, 1985, pp. 37-39.
23. TOUW-LANGENDIJK, E. M. J. and NAAFS, B. Relapse in leprosy after release from control. Lepr. Rev. 50(1979)123-127.
24. VAN BRAKEL, W., KIST, P., NOBLE, S. and O'TOOLE, S. Relapses after multidrug therapy for leprosy: a preliminary report of 22 cases in west Nepal. Lepr. Rev. 60(1989)45-50.
25. WARNDORFF VAN DIEPEN, T. and MENGISTU, G. Effect of short term supplementary treatment of the overall relapse rate and the incidence of dapsone resistant leprosy in lepromatous patients. (Abstract) Int. J. Lepr. 52 Suppl.(1984)710.
26. WATERS, M. F. R., RIDLEY, D. S. and RIDLEY, M. J. Clinical problems in the initiation and assessment of multidrug therapy. Lepr. Rev. 57 Suppl.3(1986)92-100.
27. WATERS, M. F. R. The chemotherapy of leprosy. In: The Biology of the Mycobacteria Volume 3; Clinical Aspects of Mycobacterial Disease. Ratledge, C., Stanford, J. and Grange, J. M., eds. London: Academic Press, 1989, pp. 406-473.
28. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
29. WHO STUDY GROUP. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
30. WHEATE, H. W. Six months MDT for paucibacillary leprosy: nerve damage and relapse. (Letter) Lepr. Rev. 57(1986)179.
1. M.D., Medical Superintendent, Lok Seva Sangam, D/l Everard Nagar, Sion Trombay Road, Bombay 400022, Maharashtra, India.
2. M.D., D.V.&D., D.D.V., Lok Seva Sangam, D/l Everard Nagar, Sion Trombay Road, Bombay 400022, Maharashtra, India.
3. M. S. Kini, M.B., B.S., Lok Seva Sangam, D/l Everard Nagar, Sion Trombay Road, Bombay 400022, Maharashtra, India.
4. D.V.&D., F.C.P.S., Emeritus Professor of Dermatology, L.T.M.M. College and L.T.M.G. Hospital, Sion, Bombay 400022, Maharashtra, India.
Received for publication on 24 May 1989.
Accepted for publication in revised form on 10 October 1989.