- Volume 58 , Number 1
- Page: 26–30
IgM serum antibodies to phenolic glycolipid-l and clinical leprosy: two years' observation in a community with hyperendemic leprosy
ABSTRACT
A village population with hyperendemic leprosy in Papua New Guinea was repeatedly examined for clinical leprosy and for serum IgM antibodies to phenolic glycolipid-I (APGL-I) over 2 years between 1984 and 1986. In 1984, serum APGL-I was elevated in 15% of the subjects without clinical leprosy, and the prevalence of seropositivity was not significantly different in subjects from households with or without leprosy. In 1986, the prevalence of elevated scrum APGL-I in leprosy-free subjects had risen to 23%. The incidence of seroconversion from APGL-I negative to APGL-I positive was 9.5% per year (95/1000 person years) in 253 subjects tested in 1984 and 1986. During the same period, 27 of 40 (67%) leprosy-free subjects reverted from positive to negative. The positive seroconversion rate in the community was higher than the incidence of clinical leprosy (11.2/1000 person years) over the However, elevated serum APGL-I was not associated with clinical disease and failed to predict the development of disease over 2 years. The significance of persistent seropositivity found in 14 (5%) leprosy-free subjects is uncertain.
RÉSUMÉ
Au cours de deux années, entre 1984 et 1986, on a procede à des examens cliniques pour la lèpre et à des dosages des anticorps IgM à 1'antigène phénoglycolipidique-I (APGL-I) dans le sérum, chez les habitants d'un village de Papouasie Nouvelle-Guinée ayant une prevalence três élevée de lèpre. En 1984, les taux sériques d'APGL-I étaient élevés chez 15% des sujets ne présentant pas de signe clinique pour la lèpre; la prévalence de la séropositivité n'était pas significativemcnt différente chez des individus en contact domiciliaire avec des malades de la lèpre, et chez les autres. En 1986, la prevalence de taux élevés d'APGL-I sérique chez les individus indemnes de lèpre était montée à 23%. L'incidence de virage sérique pour APGL-I de négatif à positif, était de 9,5% par an (95/1000 personnes année) chez les 253 personnes étudiées en 1984 et revues en 1986. Au cours de la même période, 27 des 40 individus sans signes cliniques de lèpre (67%) ont témoigné d'un virage de positif à négatif. Le taux de séroconversion positive dans cette communauté était plus élevé que l'incidence de la lèpre clinique (11,2/1000 personne-années) au cours de la même période. Toutefois, l'élévation des taux sériques d'APGL-I ne s'est pas révélée être un indice de prédiction pour le développement de la maladie au cours des deux années suivantes, car il n'était pas associé avec l'infection clinique. La signification d'une séropositivité persistante, observée chez 14 (5%) des individus sans lèpre, reste à expliquer.
RESUMEN
Entre 1984 y 1986, la población de una region con lepra hiperendémica en Papua, Nueva Guinea, se examino repetidamente para buscar evidencias clínicas de lepra y anticuerpos séricos contra el glicolípido fenólico-1 (AGLF I). En 1984, los AGLF-I estuvieron elevados en cl 15% de los sujetos sin lepra clinica, y la prevalencia de seropositividad no fue significativamente diferente en sujetos convivientes con lepra. En 1986, la prevalência de AGLF-I elevados en sujetos libres de lepra aumento al 23%. La incidencia, de seroconversión de AGLF-I negativos a AGLF-I positivos, fue de 9.5% por año (95/1000 personas/año) en 253 sujetos estudiados entre 1984 y 1986. Durante el mismo periodo, 27 de 40 (67%) sujetos libres de lepra revirtieron de positivos a negativos. El grado de seroconversion positiva en la comunidad fue más alta que la incidencia de lepra clinica (11.2/1000 personas/ año) en el mismo período. Sin embargo, la elevación en los AGLF-I séricos no estuvo asociada con la enfermedad clínica y no sirvió para predecir el desarrollo de la enfermedad en los 2 anos de estudio. Es incierto el significado de la serpositividad persistente encontrada en 14 (5%) dc los sujetos libres de lepra clínica.
Serum IgM antibodies to phenolic glycolipid-I (APGL-I), an antigen specific to Mycobacterium leprae, have been shown to be elevated in many patients with leprosy and in their contacts (3, 4, 9). Furthermore, it is suggested that elevated levels will precede the diagnosis in most cases, although the extent of seropositivity in endemic areas is uncertain (4, 7). As part of a longitudinal study of hyperendemic leprosy in a Papua New Guinean village, we have measured serum APGL-I and have been unable to correlate elevated levels with the presence of clinical leprosy, with the development of clinical leprosy over a 2-year period or with positive skin smears.
MATERIALS AND METHODS
The surveys. Between 1984 and 1986 the population of Kalo village on the south coast of Papua New Guinea was studied. The initial survey has been described elsewhere (1, 2), and the methods used are summarized here. All villagers were included; informed consent was obtained; personal, household and family data were collected; subjects were questioned about previous leprosy and examined for signs of the disease; additional data were obtained from the national leprosy register. Subjects were re-examined in 1985 and 1986 (at approximately 12-month intervals), and serum APGL-I was measured again in 1986 (24-month interval). Five months after the 1986 survey, subjects with elevated serum APGL-I in 1986 were again examined, and skin smears were taken and examined in Sydney by immunofluorescence. Throughout the study, the diagnosis of leprosy in new cases, whenever possible, has been confirmed by skin smears and/or histology.
Serum APGL-I. In 1984 serum was separated from venous blood and cooled in the field. After cool shipment to Australia, it was stored at -20ºC until tested. In 1986 capillary blood samples were collected and, after separation, the sera were treated similarly to those collected in 1984. APGL-I was measured at a 1:100 dilution by an ELISA based on methods described by Brett, et al. (3) and modified by Britton, et al. (4). Positive and negative control samples were included on each test plate. The positive control was pooled sera from Nepalese patients with untreated lepromatous leprosy; the negative control was pooled sera from Australians with no known exposure to leprosy. The results are expressed as the percentage optical density (OD) of the positive control. Individual negative control samples all gave results of less than 1%. Sera collected from 33 normal Melanesians from a village close to Kalo with a low incidence of leprosy gave results ranging from 0.3% to 11.6%, with a mean of 5.6% and a standard deviation (S.D.) of 2.7% (2). In this report we have defined an OD of 20% or more of the positive control as abnormal, i.e., positive.
RESULTS
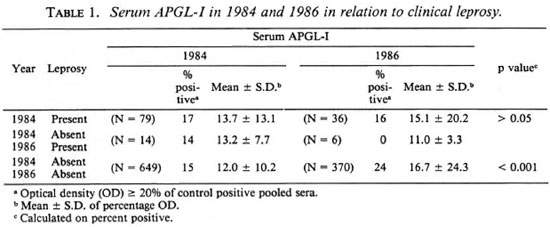
Serum APGL-I and clinical leprosy. In 1984, 960 (95%) village residents were examined and 83 (8.3%) had leprosy (including inactive disease). Between 1984 and 1986, the incidence of new cases of leprosy among subjects known to have been free of leprosy in 1984 was 11.2/1000 person years (16 cases). The diagnosis was confirmed by skin smear or biopsy in seven cases. Positive serum APGL-I did not correlate with clinical leprosy, and there was no significant difference between the mean levels of serum APGL-I in normal villagers and in patients with leprosy in 1984 or 1986 (Table 1). The serum APGL-I in 1984 was known for 14 of the 16 new cases of leprosy and was positive in two (14%). The mean value of serum APGL-I in these subjects did not differ significantly from that in subjects who did not develop leprosy (Table 1).
APGL-I in subjects without clinical leprosy
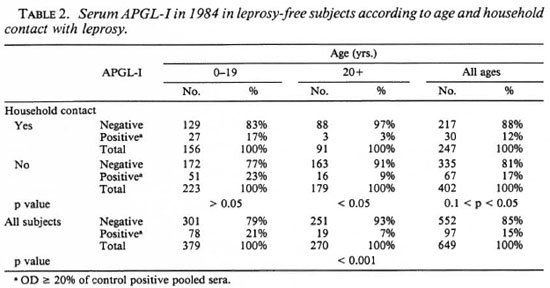
Distribution of elevated serum APGL-I in 1984. Ninety-five (15%) of the 649 subjects without clinical leprosy had elevated serum APGL-I in 1984. Seropositivity was significantly more common in subjects under 20 years of age (20.6%) than in older subjects (7%) (p < 0.001), but there was no significant difference between subjects less than 10 years old and those between 10 and 19 years old (data not shown) or between males and females (Table 2). The prevalence of elevated serum APGL-I was lower in subjects in contact with clinical leprosy in the home than in subjects without such contact, but the difference was only significant in subjects aged 20 or more years (p < 0.05) (Table 2).
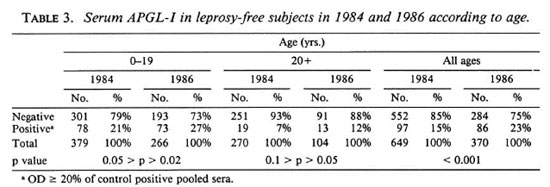
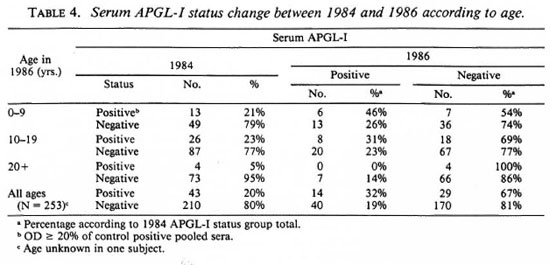
Changes in serum APGL-I between 1984 and 1986. Between 1984 and 1986 the prevalence of elevated serum APGL-I in leprosy-free subjects rose significantly from 15% to 23% (p < 0.001), and the mean value rose from 12% to 17% (p < 0.001). The increase in prevalence of elevated serum APGL-I occurred in both age groups and was statistically significant in subjects under 20 years of age (Table 3). Changes in individual serum APGL-I between 1984 and 1986 have been evaluated in 253 subjects. In 1986, 170 of 210 (81%) subjects who were negative in 1984 were still negative and 14 of 43 (33%) subjects who were positive in 1984 were still positive. Forty (19%) subjects negative in 1984 had become positive, and 26 (67%) subjects positive in 1984 had become negative (Table 4). Changes in serum APGL-I status occurred more frequently in subjects under 20 years of age (33%) than in older subjects (14%) (0.01 > p > 0.001) (Table 4).
Re-evaluation of seropositive subjects
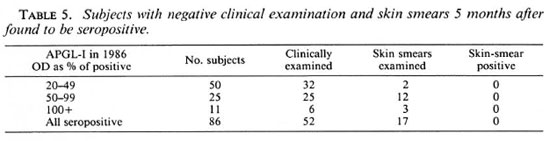
Fifty-two of 86 (60%) subjects with elevated serum APGL-I in 1986 were re-examined after 5 months and no clinical evidence of leprosy was found. Skin smears were taken from eyebrows and earlobes in the 17 subjects who consented, and these smears were negative even in three subjects with a serum APGL-I level greater than the positive control sera (Table 5).
DISCUSSION
The validity of our results depends on competent recognition of leprosy, an accurate estimation of scrum APGL-I, and a valid definition of a positive result. All subjects were examined by clinicians experienced in leprosy, and in 26 of 36 newly recognized cases the diagnosis was confirmed by skin smear and/or histology. The clinical diagnosis of leprosy may be difficult, and it is possible that a few of the unconfirmed cases did not have leprosy, but we are confident that cases of leprosy were not missed. APGL-I was measured by experienced laboratory staff using established techniques with appropriate controls and a reliable source of antigen (2). The prevalence of elevated serum APGL-I and the mean level in subjects without leprosy was higher in 1986 using capillary blood than in 1984 using venous blood (Table 1). However, capillary blood samples have been used previously (8). The mean values in patients with leprosy in 1984 were not significantly different in 1986, and individual results showed no consistent patterns of change as would be expected if there was a technical error. The criterion for a positive result is somewhat arbitrary, but the level we chose (OD 20% or more of the OD of pooled sera from patients with untreated lepromatous leprosy) is more than five standard deviations above the mean value in healthy Melanesians (2).
The lack of significant elevation of serum APGL-I in cases of leprosy diagnosed and treated before 1984 when compared to leprosy-free subjects was not unexpected, since all types of leprosy and all levels of disease activity were present in these patients who had had the disease for up to 24 years (1). However, there was no significant increase of serum APGL-I in patients with untreated disease, not even in 5 cases of multibacillary disease (1 = LL, 3 = BL, 1 = BB2). Nor was there significant elevation of serum APGL-I in 1984 in those subjects who developed leprosy within the next 2 years (Table 1). We failed to find Mycobacterium leprae in skin smears of the 12 subjects with serum APGL-I levels between 50% and 99% of the pooled positive control sample or in three subjects with values above 100% (Table 5). Our results apply only to this community where leprosy is hyperendemic but in this situation serum APGL-I has not proved useful in identifying clinical leprosy or over 2 years in predicting individuals likely to develop clinical leprosy.
Positive serum APGL-I in the absence of clinical leprosy probably indicates subclinical infection (7). Based on this presumption, our data show that subclinical infection was more common in people under 20 years of age and most seroconversions from negative to positive (82%) occurred in that age group. However, a positive serum APGL-I was not more frequent (and may have been less frequent) in household contacts of leprosy. In this village, people with leprosy are not ostracized and the way of life offers ample opportunity for transmission of air-borne infections throughout the community. Furthermore, leprosy has been endemic in the village for many years, and most subjects were life-long residents of the village and would have been exposed to M. leprae from a young age (1). This pattern of distribution of elevated serum APGL-I is compatible with widespread transmission of M. leprae with a high incidence of subclinical infection at a young age and subsequent reduced susceptibility to further infection. Presuming that positive seroconversion reflects subclinical infection, the incidence of subclinical infection between 1984 and 1986 (9.5% per year) was higher than the incidence of clinical disease (11.2/ 1000 person years) during the same period. Similar discrepant findings have been reported in household contacts (7).
Over the same 2-year period, 67% of those without clinical leprosy who were seropositive initially became seronegative. Serum APGL-I levels are directly related to the bacillary load (6) and in asymptomatic infection without treatment, falling levels may reflect an effective defense response to a subclinical infection. However, 14 (5.5%) leprosy-free subjects, all of whom are under 20 years of age, have had elevated scrum APGL-I and remained positive for at least 2 years (Table 4). This could signify latent or progressive subclinical infection, but a longer period of surveillance is required to determine the significance of persistent seropositivity. Buchanan, et al. (5) reported the development of leprosy in 2 of 18 household contacts with persistent seropositivity, and found no cases among 94 contacts who were persistently negative (50) or only transiently positive (44) followed for over 2½ years. If persistent seropositivity proves to be associated with a high risk of clinical disease then treatment before clinical manifestations becomes feasible. The development of a simple spot test for screening for APGL-I (10) would make it possible to monitor serum APGL-I in high-risk groups in endemic areas in countries where facilities are limited.
Conclusions. Elevated scrum APGL-I is common in this community with hyperendemic leprosy, particularly in younger people, but it does not identify clinical leprosy, individuals at high risk of clinical disease, or household contacts. Seroconversions are documented, suggesting a high incidence of subclinical and self-limiting infection. The small proportion of subjects who remained seropositive over 2 years may prove to be an identifiable subgroup at particular risk of disease.
Acknowledgments. We wish to acknowledge assistance from the Department of Health of the Central Province, Papua New Guinea; the Department of Community Health of the University of Papua New Guinea; the Departments of Histopathology and Microbiology of the Prince of Wales Hospital, Sydney, Australia, and the Councilors and people of Kalo. RJC was supported by a National Health and Medical Research Council Postgraduate Medical Scholarship during the work described in this manuscript.
REFERENCES
1. BAGSHAWE, A., DEBURGH, S., FUNG, S. C, CHUAH, J. and BERRY, G. The epidemiology of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 83(1989)121-127.
2. BAUMGART, K., BRITTON, W., BASTEN, A. and BAGSHAWE, A. Use of phenolic glycolipid anti-gen-I for serodiagnosis of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81(1987)1030-1032.
3. BRETT, S. J., DRAPER, P., PAYNE, S. N. and REES, R. J. W. Serologic activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
4. BRITTON, W. J., GARSIA, R.J. and BASTEN, A. The serological response to the phenolic glycolipid of Mycobacterium leprae in Australian and Nepali patients. Aust. N.Z. J. Med. 17(1987)568-573.
5. BUCHANAN, T. M., DISSSANAYAKE, S., YOUNG, D. B., MILLER, R. A., ACEDO, J. R., HARNISCH, J. P., KHANOLKAR, S. R. and ESTRADA-PARRA, S. Evaluation of the significance of antibodies to phenolic glycolipid of Microbacterium leprae in leprosy patients and their contacts. (Abstract) Int. J. Lepr. 51(1983)658-659.
6. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid antigen-1 from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
7. Serological tests for leprosy. (Editorial) Lancet 1 (1986) 533-535.
8. Wu, Q.-X., YE, G.-Y., ZHOU, L.-L., SHU, H.-W., LIU, Q., LI, X.-Y., MA, Z.-X. and Li, Z.-W. Determination of antibodies in dried blood from earlobes of leprosy patients by enzyme-linked immunosorbent assay-a preliminary report. Int. J. Lepr. 53(1985)565-570.
9. YOUNG, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221 (1983) 1057-1059.
10. YOUNG, D. B., FOHN, M. J., KHANOLKAR, S. R. and BUCHANAN, T. M. A spot test for detection of antibodies to phenolic glycolipid I. Lepr. Rev. 56 (1985) 193-198.
1. M.B.B.S, F.R.C.P., D.T.M.&H., School of Public Health and Tropical Medicine, Royal Prince Alfred Hospital and the University of Sydney, Camperdown 2050, New South Wales, Australia.
2. R. J. Garsia, M.B.B.S., F.R.A.C.P., F.R.C.P.A., Clinical Immunology Registrar, Royal Prince Alfred Hospital and the University of Sydney, Camperdown 2050, New South Wales, Australia.
3. K. Baumgart, Bsc.(Med.), M.B.B.S., Clinical Immunology Registrar, Royal Prince Alfred Hospital and the University of Sydney, Camperdown 2050, New South Wales, Australia.
4. L. Astbury, Bsc, Clinical Immunology Centre, Royal Prince Alfred Hospital and the University of Sydney, Camperdown 2050, New South Wales, Australia.
Reprint requests to Prof. A. Bagshawe, Department of Medicine, University of Zambia, P.O. Box 50110, Lusaka, Zambia.
Received for publication on 18 April 1989.
Accepted for publication on 3 August 1989.