- Volume 58 , Number 1
- Page: 31–8
Activation of complement by circulating immune complexes isolated from leprosy patients
ABSTRACT
Circulating immune complexes isolated from different types of leprosy sera as polyethylene glycol (PEG) precipitates were found to be efficient activators of the alternative pathway of complement. PEG precipitates from BL/LL leprosy patients and those with erythema nodosum leprosum were found to activate both the classical pathway and the alternative pathway of complement efficiently, while PEG precipitates from TT/BT leprosy patients and borderline tuberculoid patients in reaction were found to active the alternative pathway of complement but not the classical pathway. No significant differences were observed between the PEG precipitates from reactional and nonreactional TT/BT and BL/LL patients in their complement activating ability.
RÉSUMÉ
On a observé que les complexes immuns circulants isolés sous la forme de précipitat de polyéthilène glycol (PEG) provenant de malades atteints de différentes formes de lèpre, agissaient comme des activateurs efficaces des voies métaboliques alternatives du complément. Les précipitats PEG recueillis à partir de malades souffrant des formes BL/LL de lèpre ou bien de sujets atteints d'érythème noueux lépreux, activaient efficacement à la fois la voie métabolique classique et la voie alternative du complément. Par contre les précipitats PEG provenant de sujets TT/BT ou de malades atteints de lèpre tuberculoïde dimorphe en réaction, n'activaient que la voie métabolique alternative du complément; ils n'avaient pas d'action sur la voie classique. Aucune différence significative n'a été constatée entre les précipitats PEG provenant de malades TT/BT ou BL/LL, en réaction ou sans réaction, quant à leur capacité d'activer le complément.
RESUMEN
Se eoncontró que los complejos inmunes circulantes aislados del suero de diferentes tipos de lepra por precipitación con polietilén glicol (PEG), fueron eficientes activadores de la vía alterna del complemento. Los precipitados-PEG de pacientes con lepra BL/LL y de pacientes con eritema nodoso leproso activaron eficientemente tanto la vía clásica como la vía alterna del complemento. Los precipitados-PEG de pacientes con lepra TT/BT y BT en reacción, activaron solo la vía alterna pero no la vía clásica del complemento. No se observaron diferencias significativas entre los precipitados-PEG de pacientes TT/BT y BL/LL reaccionales y no reaccionales, en su capacidad de activar al complemento.
Elevated levels of circulating immune complexes (CICs) have been reported in almost all types of leprosy patients (2, 9, 13, 16) and deposition/in situ formation of immune complexes (ICs) is thought to be a precipitating factor for the development of the erythema nodosum leprosum (ENL) type of reaction in lepromatous (LL) leprosy patients (25). Although increased levels of CICs have been reported in all types of leprosy patients, the pathophysiological role of these complexes in leprosy has not been studied. However, Saha and his colleagues (20) have shown that CICs isolated from leprosy patients were able to activate complement.
Laboratory and animal studies have shown that ICs formed in in vivo conditions activate the complement system through both the classical pathway and the alternative pathway (3). Miller and Nussenzweig(12) have shown that activation of complement leads to solubilization of ICs, and once fully solubilized, ICs lose their ability to activate complement further and they are termed end-stage complexes (1, 8, 12, 23).
A recent report by Ramanathan, et al. (17) has shown that reactional leprosy patients of the tuberculoid and borderline tuberculoid (TT/BT) and lepromatous (BL/LL) types have a reduced complement-mediated solubilization (CMS) capacity. The total complement functional activity in these patients, however, was found to be normal. Whether reduced CMS ability plays any important role in determining the pathophysiological nature of CICs in reactional leprosy patients is not known. Furthermore, whether CICs in reactional and nonreactional leprosy patients differ in their immunopathological properties is also not known. Since complement activation leads to the generation of a variety of inflammatory reactions, the present study was planned in order to find out a) whether CICs isolated from the sera of various types of leprosy patients have the ability to activate complement, and b) whether reactional and nonreactional TT/ BT and BL/LL patients differ in their ability to activate complement pathways.
MATERIALS AND METHODS
Reagents. Polyethylene glycol (PEG 6000), bovine serum albumin (BSA), and ethylene glycol-bis (amino-ethylether) tetra-acetic acid (EGTA) were obtained from Sigma Chemical Co., Poole, Dorset, U.K.
Antigen. The cell-free extract (CFE) of Mycobacterium leprae derived from armadillo was kindly supplied by Dr. R. J. W. Rees, National Institute for Medical Research, London, from the IMMLEP (WHO) Bank.
Antisera. Anti-sheep hemolysin was obtained from Span Diagnostic, Surat, India. Anti-human IgG and IgM peroxidase conjugates were obtained from Sigma Chemical Co., St. Louis, Missouri, U.S.A.
Buffers and substrate. Phosphate buffered saline (PBS, 0.15 M, pH 7.2) and PBS containing EDTA (0.01 M EDTA, pH 7.6) were used for precipitating CICs from the serum samples. Veronal buffered saline, pH 7.35, 0.15 M containing 0.1% gelatin, 0.00015 M Ca2 and 0.005 M+ Mg2+ (GVBS++) was used for the total hemolytic complement assay (CH50). GVBS containing 0.005 M Mg 2 + and 0.05 M EGTA (GVBSMgEGTA) was used for the alternative pathway hemolytic complement assay (AH50). GVBS containing 0.01 M EDTA (GVBSEDTA) was used for stopping the complement reactions. PBS containing 0.05% Tween 20 (PBST20) was used for washing the ELISA plates. Ten mg of O-phenylene diamine and 15 μl of 6%H2O2 were mixed in 25 ml of 0.1 M citrate phosphate buffer, pH 5.0, and used as the substrate.
Sera. For the isolation of CICs, 10 ml blood samples from each of 80 leprosy patients20 tuberculoid/bordcrline tuberculoid (TT/BT); 20 BT with reaction (BTR); 20 lepromatous (BL/LL); and 20 BL/LL with ENL reaction were collected. The patients were classified by the criteria of Ridley and Jopling (18). Those patients who gave no history of reactional episodes during the preceding 30 months at the time ofblood collection were categorized as nonreactional cases. BL/LL patients who had episodes of reactions during the preceding months, and those who were having ENL and TT/BT patients in reaction at the time of blood collection were designated as reactional cases. Blood samples collected from 15 healthy laboratory volunteers from the Central JALMA Institute for Leprosy, Agra, were used as control samples. All ofthe sera obtained from these blood samples were stored at -70ºC in 0.5 ml aliquots.
Isolation of CICs by PEG precipitation. CICs were precipitated by 2.5% (w/v) PEG 6000 following the method ofCreighton. et al, (7). The PEG precipitates were freshly prepared for each experiment from aliquots of serum samples for each group. One ml of the aliquots was diluted 1:5 in PBS EDTA. Equal volumes of 5% PEG 6000 in PBS were added to the diluted sera. After 1824 hr incubation at 4ºC, the precipitates were separated by centrifugation at 1032 x g x 20 min at 10ºC. The precipitates were washed once with 2.5% PEG. They were then thoroughly dissolved in PBS at 37ºC for 2 hr, and the volume was reconstituted to the original serum volume with PBS. All PEG precipitates were stored at -20ºC in 0.5 ml aliquots and were thawed once and used in the experiments.
Estimation of total protein content. Total protein concentrations in the PEG precipitates were estimated using Lowry, et al.'s method (10).
Estimation ofmycobacterial CICs in PEG precipitates. The CICs in the PEG precipitates containing IgG and IgM antibodies directed against M. leprae antigens were determined using an enzymelinked immunosorbent assay (ELISA) developed in our laboratory. The ELISA was carried out as follows: 50 μl of 100 μg/ml cellfree extract of M. leprae (CFE) was added to each well of a 96well microliter plate (Ubottom; Nunc, Denmark). After overnight incubation at 4ºC, the antigen solution was removed and the plates were washed twice with PBST20. The plates were then incubated at 37ºC for 2 hr after adding 100 μl of 1% BSA in PBS to block all free sites of each well. After washing the wells twice with PBST20, 50 μl of 1/10 diluted PEG precipitates from various types of leprosy patients and normal controls were added to the wells, and the plates were incubated for 2 hrat 37ºC. The PEG precipitates were also added to the 1% BSAPBScoated wells in addition to the CFEcoated wells to determine the specific as well as nonspecific binding of the CICs to the M. leprae antigens. After washing the wells with PBST20 five times, 50 μl of 1:1000 peroxidaseconjugated antihuman IgG or IgM was added to all the wells, and the plates were incubated for 2 hr at 37ºC. The plates were finally washed five times with PBST20, and 50 μl ofthe substrate was added to each well. The plates were incubated for 30 min in the dark, and the reaction was stopped by the addition of 50 μl of7% H2SO4. The intensity of the final color reaction was read at 492 nm in a Titertck Multiskan plus ELISA reader (Flow Laboratories, U.K.).
Determination of optimal dose of PEG precipitates for activation of complement. For the classical pathway (CP) of complement activation, 0.025 ml fresh normal human serum (NHS) wasdiluted with GVBS++ buffer in the ratio of 1:100. PEG precipitates and the diluted NHS were mixed in the proportions of 1:1, 2:1, 3:1, and 4:1 and were incubated at 37ºC for 1 hr.
For the alternative pathway (AP) of complement activation, 0.065 ml NHSMg-EGTA (0.05 ml NHSand0.005 MgCl2 and 0.01 ml 50 mM EGTA) was diluted with GVB-SMgEGTA in the ratio of 1:13.5. The PEG precipitates and the diluted NHS were mixed in the proportions of 1:1, 2:1. 3:1, 4:1, 5:1, and 6:1, and were incubated at 37ºC for 1 hr.
Determination of CH50 consumption. The PEG-prccipitate-trcated NHS samples were subjected to the residual CH50 assay using the method of Mayer (11). Briefly, serial dilutions of PEG-precipitate-treated NHS were incubated with 0.1 ml of 2.5% optimally sensitized sheep red blood cells (SRBC) at 37ºC for 1 hr, followed by the addition of 2 ml of GVBSEDTA in each dilution to stop further activation of complement. Nonlysed SRBC were spun down at 600 X g X 7 min. The percent hemolysis was measured in the supernatant for each dilution at 412 nm in a spectrophotometer (Shimadzu UV-160, Kyoto, Japan).
Determination of AH50 consumption. The PEG-precipitate-treated NHS samples (containing GVBSMgEGTA) were assayed for the residual AH50 activity using the method of Platts-Mills and Ishizaka (14). Briefly, serial dilutions of PEG-precipitatetreated NHS were incubated with 0.05 ml of 2.5% washed rabbit red blood cells (RRBC) at 37ºC for 1 hr, followed by the addition of 1 ml of GVBSEDTA in each dilution to stop further activation of complement. Nonlysed RRBC were spun down at 660 x g x 7 min. The percent hemolysis in the supernatant was measured for each dilution at 412 nm in a spectrophotometer as above.
Statistical analysis. Group means were compared by Student's t test. The total hemolytic complement assay (CH50 U/ml) and hemolytic assay for alternative pathway (AH50 U/ml) were calculated by linear regression analysis. Program 6D of the BMDP statistical software (Dixon and Brown, 1987) was used to calculate the correlation coefficient and simple linear regression (6).
RESULTS
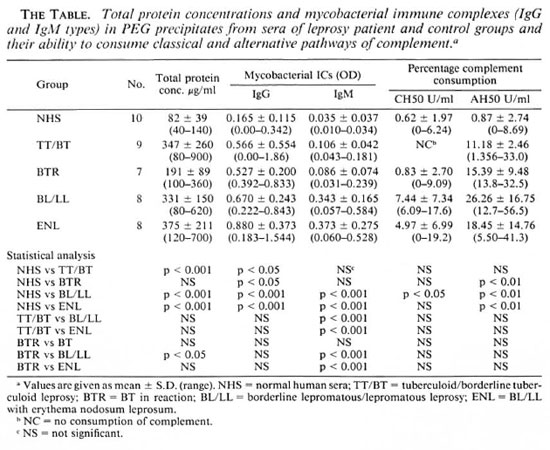
Total protein content in PEG precipitates. It can be seen from The Table that the total protein concentrations in the PEG precipitates from TT/BT, BL/LL, and ENL patients were significantly higher than those in the control group. Among the PEG precipitates from the patient groups, it was found that the BL/LL group had a significantly higher protein concentration than did the BTR group. However, no marked differences in protein concentration were observed among the TT/BT, BL/LL, and ENL groups.
Immune complex levels in PEG precipitates. The Table shows that the PEG precipitates from the BL/LL and ENL groups had significantly higher levels of IgG and IgM antimycobacterial antibodies than did those from the TT/BT, BTR, and control groups. The PEG precipitates from TT/BT, BTR, and the control groups had very low IgM levels. However, the IgG level was found to be high in one of the BT patients. No significant differences in the levels of IgG and IgM types of immune complexes were noticed between the reactional and nonreactional cases of either TT/BT or BL/LL types of leprosy.
Correlation between levels of total proteins and mycobacterial immune complexes. A significant positive correlation was found between the values of total proteins and the mycobacterial ICs (IgG and IgM type) in the PEG precipitates. The correlation coefficients and the p values obtained were r = 0.750, p < 0.001 and r = 0.646, p < 0.001 for IgG and IgM (Figs. 1 and 2).
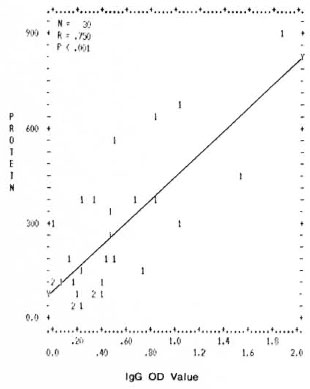
Fig. 1. Correlation between levels of total protein concentration (μg/ml) and immune complexes (IgG type) in PEG precipitates from leprosy patients and normal controls. Numbers denote the number of identical results.
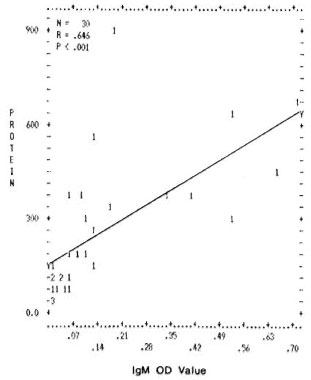
Fig. 2. Correlation between levels of total protein concentration (μg/m1) immune complexes (IgM type) in PEG precipitates from leprosy patients and normal controls. Numbers denote the number of identical results.
Dose-dependent effect of PEG precipitates on complement. In our initial experiments to determine the optimal dose of PEG precipitates in activating complement, the different concentrations of PEG precipitates obtained from only the BL/LL groups were tested for their ability to activate the classical and alternative pathways. Figure 3a shows that while PEG precipitates from BL/LL sera were capable of activating the classical pathway at twofold concentration and above, the PEG precipitates from NHS could do so only at fourfold concentration. As for activating the alternative pathway, Figure 3b shows that while the PEG precipitates from NHS failed to activate this pathway at any concentration, PEG precipitates from the BL/LL sera could activate the alternative pathway at all concentrations. Hence, for subsequent experiments for a complement consumption study, we used the PEG precipitates from the various groups of subjects at a twofold concentration for CH50 and at an equal (1:1) concentration for AH50.
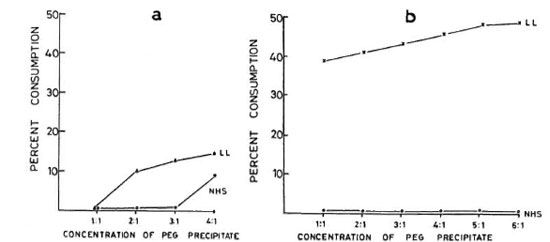
Fig. 3.a = Normal human serum (NHS) (25 μl) diluted to 1:100 in GVBS++ was incubated at 37ºC for 1 hr with 25 pl, 50 μl, 75 μl, and 100 μl of PEG precipitates from NHS and LL sera. The effects of different concentrations of PEG precipitates from NHS (O-----O) and LL (Δ-----Δ) sera on the classical pathway of complement are shown. b = NHS (50 μl), containing 5 μl 5 mM MgCI2 and 10 μl 50 mM EGTA, diluted to 1:13.5 with GVBSMgEGTA was incubated at 37ºC for 1 hr with 50 μl, 100 μl, 150 μl, 200 μl, 250 μl, and 300μl of PEG precipitates from NHS and LL sera. The effects of different doses of PEG precipitates obtained from NHS (O-----O) and LL (×-----×) sera on the alternative pathway of complement are shown.
CH50 consumption. As can be seen in The Table, although the PEG precipitates from BL/LL patients can activate the classical pathway of complement significantly, PEG precipitates from the TT/BT and BTR patients and the control group were found to be inefficient activators of this pathway. The percentage complement consumption patterns obtained were: TT/BT, 0%; BTR, 0.83% ± 2.70; BL/LL, 7.44% ± 7.34; and ENL, 4.97% ± 6.99. PEG precipitates from one NHS were also found to activate the classical pathway (6.24%). The difference in the mean values of percentage consumption between NHS and BL/LL was found to be statistically significant (p < 0.05); whereas the differences between NHS and BTR or ENL were not found to be statistically significant (p > 0.1; p > 0.05). No direct hemolytic effect of the PEG precipitates on the sensitized SRBC under similar test conditions was observed.
AH50 consumption. From The Table it can be seen that the PEG precipitates from all of the leprosy groups were found to activate the alternative pathway of complement efficiently. The percentage consumption patterns obtained were: TT/BT, 11.18% ± 2.46; BTR, 15.39% ± 9.48; BL/LL, 26.26% ± 16.75; and ENL, 18.45% ± 14.76. While the differences in the percentage consumption between the control and individual leprosy groups were statistically significant (i.e., NHS vs BTR, BL/LL, and ENL = p < 0.01, 0.01, and 0.01), the differences within the leprosy groups were not found to be statistically significant (p > 0.05). The PEG precipitate from one normal serum was also found to activate the alternative pathway to a small extent (8.69%).
DISCUSSION
It is now well established that all types of leprosy patients have elevated levels of CICs, and this is more evident in BL/LL and ENL patients (2, 13, 16, 22, 25). The immunochemical analysis of these CICs isolated in the form of PEG precipitates showed the presence of IgG, IgM, IgA, C1q, C3, C4, C-reactive protein, and rheumatoid factor (5, 16). Recently, Ramanathan, el al. (17) and Chakrabarty, et al. (4) have further reported diminished complement-mediated solubilization of in vitro formed immune complexes in the sera of BTR, BL/LL, and ENL patients. Whether the reduced complementmediated solubilization capacity of reactional and nonreactional patients' sera actually renders the CICs unsolubilized in these patients, and therefore causes harmful effects, is not known.
This study is an attempt to find out whether or not immune complexes in the circulation of different types of leprosy patients arc different in their ability to activate the complement system in order to better understand the role of immune complexes in leprosy pathology. In the present study, a positive correlation between the protein contents and the levels of immune complexes might indicate (Figs. 1 and 2) that the high levels of total protein in the PEG precipitates are due to an increase in immune complex levels.
Saha, et al. (20) have demonstrated the complement-activating ability in PEG precipitates from leprosy patients for the classical pathway. Like Saha, et al. (20), we also could not demonstrate any difference in the complement-activating ability of the PEG precipitates obtained from reactional and nonreactional patients. However, their study included only LL and ENL types of leprosy. Our results show that while the PEG precipitates from sera of BL/LL and ENL patients were capable of activating both the classical and the alternative pathways of complement, PEG precipitates from TT/BT and BTR sera were found to activate the alternative pathway more efficiently than the classical pathway of complement. This is probably due to the fact that CICs isolated as PEG precipitates were found to contain mycobacteria (19), and mycobacterial components arc known to activate the alternative pathway of complement (15, 21).
The differences in the total protein concentration in the PEG precipitates from TT/BT, BL/LL, and ENL patients were not found to be statistically significant. The fact that the total protein concentration in the PEG precipitates from TT/BT patients was found to be as high as that of the PEG precipitate from ENL sera is very difficult to explain. However, it is known that BT leprosy is a very heterogeneous group in which it is common to find a large variation in antibody levels (24). The present study also revealed a wide range of protein levels in the PEG precipitates in this group. Probably due to this range of variation in protein values in PEG precipitates, there is no statistical difference in the protein values between the TT/BT and ENL groups.
The differences in the IgG CIC levels in the PEG precipitates from TT/BT, BL/LL, and ENL sera were not found to be statistically significant. However, the complement activating ability through both the classical and the alternative pathways was found to be much higher in the PEG precipitates from the BL/LL and ENL groups than in the PEG precipitates from TT/BT and BTR sera; the reason for this could be the presence of high levels of mycobacterial IgM types of immune complexes in these patients (The Table). Further, in all of the leprosy and control groups, a positive correlation was found between the immune complex levels in the PEG precipitates and their ability to activate the classical and the alternative pathways of complement (Figs. 4 and 5). It was interesting to note that while the normal human serum PEG precipitates were unable to activate complement up to a threefold concentration for the classical pathway and up to a sixfold concentration for the alternative pathway, PEG precipitates from the BL/LL patients were found to activate the classical pathway at a twofold concentration and above, and the alternative pathway at all concentrations. This might indicate that the CICs were not completely solubilized and therefore were available in the BL/LL patients for further activation of the complement system.
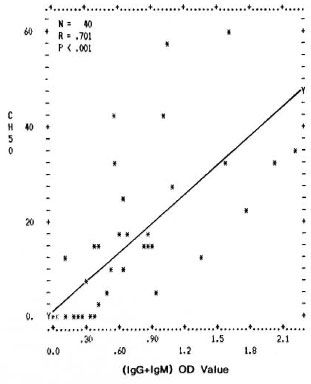
Fig. 4. Correlation between levels of mycobacterial immune complexes (IgG + IgM) in PEG precipitates and their classical pathway complement (CHSO U/ml) consumption capacity. Y axis represents percentage (CH50 U/ml) consumption; X axis denotes combined (OD492) value for (IgG + IgM).
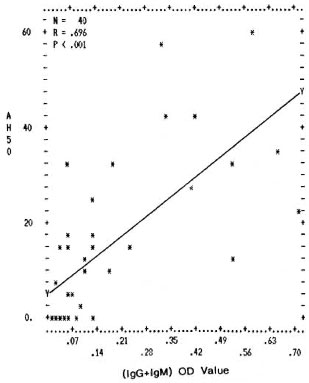
Fig. 5. Correlation between levels of mycobacterial immune complexes (IgG + IgM) in PEG precipitates and their alternative pathway complement (AH50 U/ml) consumption capacity. Y axis represents per-centage (AH50 U/ml) consumption; X axis denotes combined (0D492) value for (IgG + IgM).
We can conclude from this study that circulating immune complexes isolated from TT/BT and BL/LL leprosy sera were functionally different from each other with regard to their complement activating ability. However, no significant functional difference was noticed between the PEG precipitates from reactional and nonreactional types of TT/BT and BL/LL leprosy groups.
Acknowledgments. We thank Mr. P. N. Sharma, Mr. C. Pal, Mr. Kuldeep K. Kulshreshta, and Mr. Malikhan Singh for their technical assistance. We also thank Mr. Anil Kumar Chopra for secretarial help and Mr. Hari Om for photographic help. We gratefully acknowledge the help of Dr. H. Srinivasan, Director of the Institute, in reviewing the manuscript.
REFERENCES
1. BAATRUP, G., PETERSEN, I., JENSEMERS, I. C. and SVEHAG, S. E. Reduced complement mediated immune complex solubilizing capacity and the presence of incompletely solubilizcd immune complexes in sera. Clin. Exp. Immunol. 54(1983)439-447.
2. BJORVANTN, B., BARNETSON, R. St. C, KRONVALL, G. K., ZUBLER, R. H. and LAMBERT, P. H. Immune complexes and complement hypcrcatabolism in patients with leprosy. Clin. Exp. Immunol. 26(1976)388-396.
3. CASALI, P., PERRIN, L. H. and LAMBERT, P. H. Immune complexes and tissue injury. In: Immunological Aspects of Infectious Diseases. Dick, G., ed. Lancaster, England: Falcon House, 1979, 295-342
4. CHAKRABARTY, A. K., KASHYAP, A., SEHGAL, V. N. and SAHA, K. Solubilization of preformed immune complexes in sera of patients with type 1 and type 2 lepra reactions. Int. J. Lepr. 56(1988)559-565.
5. CHAKRABARTY, A. K., MAIRE, M., SAHA, K. and LAMBERT, P. H. Identification of components of IC purified from human sera. II. Demonstration of mycobacterial antigens in immune complexes isolated from sera of lepromatous patients. Clin. Exp. Immunol. 51(1983)225-231.
6. CHASEN, S. P6D bivariatc(scatter) plots. In: BMDP Statistical Software. Dixon, W. J. and Brown, M. B., eds. Berkeley: University of California Press, 1987, pp. 133-141.
7. CREIGHTON, W. D., LAMBERT, P. H. and MIE- SCHER, P. A. Detection of antibodies and soluble antigen complexes by precipitation with polyeth ylene glycol. J. Immunol. 111(1973)1219-1227.
8 CZOP, J. and NUSSENZWEIG, V. A. Studies on the mechanism of solubilization of immune precipi tates by serum. J. Exp. Med. 143(1976)615-630.
9. GELBER, R. H., DRUTZ, D. J., EPSTEIN, W. V. and FASAL, P. Clinical correlates of CI q, precipitating substances in the sera of patients with leprosy. Am. J. Trop. Med. Hyg. 23(1974)471-475.
10. LOWRY, O. H., ROSEBROUGH, N. J., FARR, A. L. and RANDALL, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chcm. 193(1951)265-275.
11. MAYER, M. M. Complement and complement fixation. In: Experimental immunochemislry. 2nd ed. Kabat, E. A. and Mayer, M. M., eds. Springfield, Illinois: Charles C Thomas, 1961, pp. 133-240.
12. MILLER, G. W. and NUSSENZWEIG, V. A new complement function: solubilization of antibody-antigen aggregates. Proc. Natl. Acad. Sci. U.S.A. 72(1975)418-122.
13. MORAN, C. J., RYDER, G, TURK, J. L. and WATERS, M. F. Evidence for circulating immune complexes in lepromatous leprosy. Lancet 2(1972)572-573.
14. PLATTS-MILLS, T. A. E. and ISHIZAKA, K. Activation of the alternate pathway of human complement by rabbit cells. J. Immunol. 113(1974)348-358.
15. RAMANATHAN, V. D., CURTIS, J. and TURK, J. L. Activation of the alternative pathway of complement by mycobacteria and cord factor. Infect. Immun. 29(1980)30-35.
16. RAMANATHAN. V. D., PRAKASH, O., RAMU, G, PARKER, D., CURTIS, J., SENGUPTA, U. and TURK, J. L. Isolation and analysis of circulating immune complexes in leprosy. Clin. Immunol. Immunopathol. 32(1984)261-268.
17. RAMANATHAN, V. D., SHARMA, P., RAMU, G. and SENGUPTA, U. Reduced complement mediated immune complex solubilization in leprosy patients. Clin. Exp. Immunol. 60(1985)533-558.
18. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
19. SAHA, K., CHAKRABARTY, A. K. and PRAKASH, N. A quick method of demonstrating bacillacmca in patients with lepromatous leprosy and ultrastructural studies of the circulating acid fast bacilli. Trans. R. Soc. Trop. Med. Hyg. 77(1983)660-664.
20. SAHA, K., CHAKRABARTY, A., SHARMA, V. K. and SEHGAL, V. N. Polyethylene glycol precipitates in scrum during and after erythema nodosum leprosum-study of their composition an mentary activity. Int. J. Lepr. 52(1984)44-48.
21. SAHA, K., SHARMA, V., CHAKRABARTY, A. K. and SEHGAL, V. N. Breakdown product of factor B as an index of complement activation in lepromatous leprosy and its relation with bacillary load. Scand. J. Immunol. 17(1983)37-43.
22. SHWE, T. Immune complexes in glomeruli of patients with leprosy. Lepr. Rev. 42(1972)282-289.
23. TAKAHASHI, M., TAKAHASHI, S. and HIROSE, S. Solubilization of antigen-antibody complexes: a new function of complement as a regulator of immune reactions. Prog. Allergy 27(1980)134-166.
24. TOUW LANGENDIJK, E. J. M., WARNDORF VAN DIEPEN, T., HARBOE, M. and BELEHU, A. Relation between anti-Mycobacterium leprae antibody activity and clinical features in borderline tuberculoid (BT) leprosy. Int. J. Lepr. 51(1983)305-311.
25. WEMAMBU, S. N. C, TURK, J. L., WATERS, M. F. R. and REES, R. J. W. Erythema nodosum leprosum a clinical manifestation of the Arthu nomenon. Lancet 2(1969)933-935.
1. M.Sc, Research Assistant, Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India. Dr. Ramanathan's present address: Tuberculosis Research Centre, Chetput, Madras, India.
2. M.B.B.S., Ph.D., Senior Research Officer, Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India. Dr. Ramanathan's present address: Tuberculosis Research Centre, Chetput, Madras, India.
3. M.D., Deputy Director, Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India. Dr. Ramanathan's present address: Tuberculosis Research Centre, Chetput, Madras, India.
4. M.D., Senior Research Officer, Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India. Dr. Ramanathan's present address: Tuberculosis Research Centre, Chetput, Madras, India.
5. M.Stat., Senior Research Officer, Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India. Dr. Ramanathan's present address: Tuberculosis Research Centre, Chetput, Madras, India.
6. M.V.Sc, Ph.D., Deputy Director, Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India. Dr. Ramanathan's present address: Tuberculosis Research Centre, Chetput, Madras, India.
Reprint requests to Mrs. Padmawati Tyagi.
Received for publication on 9 June 1989.
Accepted for publication in revised form on 11 October 1989.