- Volume 58 , Number 1
- Page: 39–43
Intraocular pressure and iris denervation in Hansen's disease
ABSTRACT
We retrospectively analyzed 255 Hansen's disease patients and found low intraocular pressure (< 7 mm Hg) in 12% of them. We showed a correlation between low intraocular pressure and avascular keratitis and iritis. We also found that patients with low intraocular pressures had abnormally large postural changes in intraocular pressure. We speculate that abnormalities in the autonomic innervation of the anterior segment of the eye may be related to the intraocular pressure abnormalities. Further investigations along this line may increase our understanding not only of the pathophysiology of Hansen's disease but also of the mechanisms regulating homeostasis of intraocular pressure.
RÉSUMÉ
Sur 255 patients souffrant de la maladie de Hansen, on a observé chez 12% d'entre eux une pression intraoculaire faible (< 7 mm Hg). Une corrélation a été constatée entre une faible pression intraoculaire d'une part, et d'autre part une kératite avasculaire ou de l'iritis. On a également relevé que les malades ayant des pressions intraoculaires faibles présentaient des modifications anormalement prononcées de cette pression oculaire lors de changements posturaux. On émet dès lors l'hypothèse que des anomalies dans l'innervation autonome du segment antérieur de l'oeil pourraient être en relation avec des anomalies de la pression intraoculaire. Des investigations supplémentaires dans ce domaine seraient utiles pour accroître notre connaissance, non seulement de la patho-physiologie de la maladie de Hansen, mais également des mécanismes qui déterminent l'homéostase de la pression intraoculaire.
RESUMEN
Analizamos retrospectivamente a 255 pacientes con la enfermedad de Hansen y encontramos una baja presión intraocular (< 7 mm Hg) en 12% de ellos. Demostramos una correlación entre baja presión intraocular y keratitis c iritis avascular. También encontramos que los pacientes con baja presión intraocular tenían cambios posturales anormalmente grandes en la presión intraocular. Especulamos que las anormalidades en la inervación autónoma del segmento anterior del ojo pueden estar relacionadas con las anormalidades en la presión intraocular. Una mayor investigación en esta línea puede mejorar nuestro conocimiento no solo de la patofisiología de la enfermedad de Hansen sino también de los mecanismos que regulan la homeostasis de la presión intraocular.
Mycobacterium leprae infiltrate the skin, cutaneous nerves, and the anterior segment of the eye, causing eyebrow and lash loss, lid nodules, corneal nerve beading, avascular keratitis, decreased corneal sensation, decreased lacrimation, and acute and chronic iridocyclitis. Low intraocular pressure has previously been reported in Hansen's disease (leprosy) (2, 8, 12). The precise responsibility for this low pressure has not been determined. We report herein our findings of the intraocular pressures and postural changes in intraocular pressure in a group of Hansen's disease patients. Because we hypothesized that intraocular pressure abnormalities might be related to ocular autonomic dysfunction, we also tested a small group of patients for ocular sympathetic dysfunction.
MATERIALS AND METHODS
Approximately 500 patients with biopsyproven Hansen's disease are registered at the San Francisco Regional Hansen's Disease Clinic, San Francisco, California, U.S.A. All patients are encouraged to undergo an eye exam by an ophthalmologist every 1-2 years. For the past 2½ years the results have been recorded on standardized examination forms and the data subsequently computerized. Some preliminary results based on 72 patients and 17 controls have been reported previously (8).
Examination includes gross examination of the lids, lashes, and brows; visual acuity testing (with and without pinhole glasses); pupillary size and response to light; Schirmer tear test; measurement of corneal sensitivity with Cochet-Bonnet anesthesiometer; slit lamp exam of cornea, anterior chamber, iris, and lens; Goldmann or Perkins applanation tonometry; and fundus examination. The standardized examination forms of the last consecutive 255 patients were analyzed to establish the distribution of intraocular pressure.
In 123 patients we conducted a case-control study measuring postural changes in intraocular pressure. The patients were examined as noted above and intraocular pressure was determined using a Perkins hand-held applanation tonometer. The procedure was performed as follows: a drop of Fluress® was instilled in each eye with the patient seated and the intraocular pressure was measured in the right and then the left eye with a Perkins tonometer. Similar measurements were made in a control group of 40 same-age volunteers, consisting of 17 first-degree family members of patients and 23 volunteers with no known eye or systemic disease. No difference was found between the family member controls and the 23 volunteers; therefore we have combined both groups into one control group.
We also tested 21 patients and 15 healthy volunteers for iris denervation. The patients and controls were selected from the study population described above without knowledge of the eye exam results on the part of the examiner. Photographs of each patient's pupils were taken under standard illumination with a 28 mm macro lens and 100 ASA film. A drop of 1% OH-amphetamine (paredrine) from a standard dropper pipette was then instilled in the right eye only. After 30 min, photographs were taken under the same conditions. The photographic slides were projected at 10 x magnification, and the pupil diameter was independently measured in millimeters by two observers. We used the pre-paredrine photograph as a control for physiologic anisocoria and the left eye as a control for change in ambient light conditions. The formula suggested by Swift and Bauschard (13) was used for calculation of our results: pupil response = (right pupil post-paredrine - left pupil post-paredrine) - (right pupil pre-paredrine - left pupil pre-paredrine).
The results of our studies were analyzed utilizing analysis of variance and chi-squared on SPSSx.
RESULTS
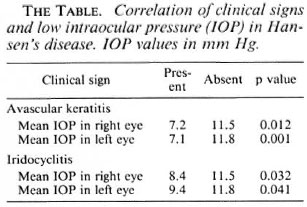
Intraocular pressure. Sixty-one of the 510 eyes (12%) had an intraocular pressure of < 7 mm. The distribution is shown in Figure 1 and compared to the intraocular pressure distribution of a general population reported by Armaly (1). Low intraocular pressure was independently associated with avascular keratitis and with iritis, either active or with signs of previous iritis (The Table).

Fig. 1. Intraocular pressure in Hansen's disease patients and general population. * = Armaly, M. F. (1).
Postural change in intraocular pressure. The mean change in intraocular pressure (upright to supine) in Hansen's disease patients was +3.24 mm Hg, as compared to +0.95 mm Hg among controls (p < 0.005) (Fig. 2). There was a negative correlation between intraocular pressure and postural change in intraocular pressure. Patients with > 4 mm Hg change in intraocular pressure had a mean of 10.76 mm Hg intraocular pressure in the upright position. Patients with a postural change of < 4 mm Hg had a mean of 12.25 mm Hg intraocular pressure in the upright position (p = 0.04). We found no correlation between abnormal clinical signs (including iridocyclitis and avascular keratitis) and large postural change in intraocular pressure. Furthermore, paucibacillary and multibacillary patients were equally likely to have large postural changes.
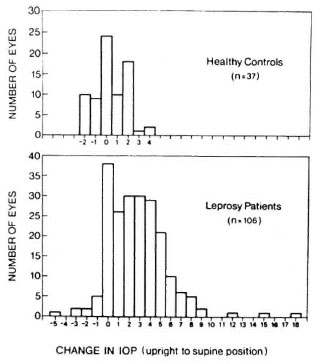
Fig. 2. Postural changes in intraocular pressure.
Iris denervation. The average pupil response to paredrine in Hansen's disease patients was 1.2 mm, compared to 1.7 mm among controls (p = 0.038). Poor pupil response to paredrine did not correlate with clinical signs of ocular Hansen's disease, low intraocular pressure, large postural changes in intraocular pressure, disease duration, or disease type.
DISCUSSION
Our findings support those of others who have reported low intraocular pressure in Hansen's disease patients. In 531 lepromatous and tuberculoid patients in Nepal, Brandt (2) found lower intraocular pressure in patients with gross evidence of iridocyclitis (posterior synechiae) as compared to those without evidence of uveal involvement. Slem (12) measured intraocular pressure in 38 eyes and performed tonography in 49 eyes in a group of Hansen's disease patients in Turkey. He concluded that low intraocular pressure was the result of decreased aqueous production. This correlates with the histopathologic reports of atrophy and hyalinization of the ciliary body in eyes of Hansen's disease patients.
These findings suggest that low intraocular pressure might serve as a predictor of iris and ciliary body involvement, and might be useful for predicting low-grade iridocyclitis in areas where slit-lamp exam is not possible.
A number of authors have reported variable findings in intraocular pressure changes with body position in healthy populations. Most of them found that the intraocular pressure rises less than 3-5 mm Hg from the upright to the supine position (3, 7, 10, 14, 15). In our studies we found that 15% of Hansen's disease patients had > 4 mm rise in pressure; whereas no healthy controls had similar changes. The mechanisms regulating homeostasis of intraocular pressure with body position changes are unclear. Because the changes in these patients occurred rapidly (immediately upon assumption of the supine position), we believe that the autonomic nervous system might be responsible. The intraocular pressure in those patients with a large rise in pressure in the supine position immediately returned to the previous level when they sat up. In normal eyes, the episcleral venous pressure appears to correlate with postural changes in intraocular pressure (6). Episcleral venous pressure may be regulated by the ocular autonomic nervous system. Thus, it is possible that abnormalities of the ocular autonomic nerve supply in Hansen's disease patients are responsible for the large postural changes in intraocular pressure seen in these patients.
Previous studies of autonomic dysfunction in the cardiovascular systems of Hansen's disease patients report conflicting results (4, 9, 11). We tested a number of our patients for orthostatic hypotension, but found no evidence of fall in blood pressure. However, minimal or no involvement of the cardiac autonomic system would be expected since these nerves are deep and at a temperature not conducive to replication of M. leprae. On the other hand, M. leprae have an affinity for the small unmyelinated nerves in the anterior segment of the eye as manifested by the beading of corneal nerves (pathognomonic of leprosy) and by avascular keratitis which represents infiltration of bacilli along nerves in the cornea and directly into the stroma.
In an effort to evaluate the role of the ocular autonomic nervous system in the pressure abnormalities of these patients, we used paredrine to look for iris denervation, ffytche has suggested that the miotic pupils seen in Hansen's disease are due to ocular sympathetic dysfunction (5). Swift and Bauschard (13) used 1% epinephrine to look for sympathetic denervation of the irises in 20 Hansen's disease patients and 20 healthy controls. Paredrine and epinephrine differ in their mechanisms of action; paredrine specifically tests the terminal neuron in the sympathetic pathway by causing release of stored norepinephrine at the nerve ending in the iris. If these sympathetic nerves to the iris are damaged, the iris responds minimally or fails to respond because of a decrease or absence of stored norepinephrine. One percent epinephrine tests for denervation supersensitivity which may result from nerve damage at any level. A potential problem with using any topically administered drug to test pupil response is the fact that corneal pathology will influence penetration of the drug into the anterior chamber. In our pupil study population there were no patients with corneal pathology, and we showed a significant difference in the pupillary response of Hansen's disease patients compared to our controls. However; conclusions based on this study are limited because our patients and controls were not matched for age and race, both of which may affect pupil response to drugs.
In order to clarify possible associations between ocular autonomic dysfunction, intraocular pressure abnormalities, and other ocular pathology, tests of ocular autonomic nerve dysfunction need to be performed in patients and matched controls. Furthermore, it will be necessary to follow longitudinally patients with intraocular pressure abnormalities in order to assess the predictive value of these abnormalities.
Acknowledgments. This work was supported in part by: the National Institutes of Health, National Eye Institute, Training Grant #EYO7058 (Dr. Lewallen); the E. A. Baker Foundation (C.N.I.B.), Canada, and the Aga Khan Foundation, Switzerland (Dr. Hussein); the World Health Organization (Dr. Courtright); the Albert and Lotte Haas Foundation, San Francisco; and the National Hansen's Disease Programs of the U.S. Public Health Service (Dr. Gelber). Special thanks to Vivian Demarest, R.N., M.P.H., for her help with the iris denervation studies.
REFERENCES
1. ARMALY, M. F. On the distribution of applanation pressures. Arch. Ophthalmol. 73(1965)11-18.
2. BRANDT, F., MALLA, O. K. and ANTEN J. G. F. Influence of untreated chronic plastic iridocyclitis on intraocular pressure in leprous patients. Br. J. Ophthalmol. 65(1981)240-242.
3. BRONNER, M. A., FRANK, H. and MARGRAFF, C. Influence de la position du corps sur le tonus oculaire et les pulsations intraoculaires. Bull. Soc. Ophathmol. France 7-8(1976)657-663.
4. DABHOLKAR, V. R. and GAITONDE, B. B. A study of autonomic functions in leprosy. Lepr. India 54(1982)303-317.
5. FFYTCHE, T. Iris in leprosy. Trans. Ophthalmol. Soc. U.K. 101(1981)325-330.
6. FRIBERG, T. R., SANBORN, G. and WEINREB, R. N. Intraocular and episcleral pressure increase during inverted posture. Am. J. Ophthalmol. 103(1987)523-526.
7. GALIN, M. A., MCIVOR, J. W. and MAGRUDER, G. B. Influence of position on intraocular pressure. Am. J. Ophthalmol. 55(1963)720-723.
8. HUSSEIN, N., COURTRIGHT, P., OSTLER, H. B., HETHERINGTON, J. and GELBER, R. H. LOW intraocular pressure and postural changes in intraocular pressure in patients with Hansen's disease. Am. J. Ophthalmol. 105(189)80-83.
9. KALE, H. D., ZAWAR, P. C, CHAWHAN, R. N. and KULKARNI, G. R. Cardiac dysautonomia in lepromatous leprosy. Indian J. Lepr. 56(1984)563-568.
10. KRIEGLSTEIN, G. K. and LANGHAM, M. E. Influence of body position on the intraocular pressure of normal and glaucomatous eyes. Ophthalmologica 171(1975)132-145.
11. SINGH, L. P.. MEHTA, S. R., GUPTA, C. M. and BHATE. R. D. Cardiovascular system in leprosy. Indian J. Lepr. 58(1986)69-72.
12. SLEM, G. Clinical studies of ocular leprosy. Am. J. Ophthalmol. 71(1971)431-434.
13. SWIFT, T. R. and BAUSCHARD, F. D. Pupillary reactions in lepromatous leprosy. Int. J. Lepr. 40(1972)142-148.
14. TARKKANEN, A. and LEIKOLA, J. Postural variations of the intraocular pressure as measured with McKay-Marg tonometer. Acta Ophthalmol. 54(1967)569-575.
15. WUTHRICH, U. W. Postural change and intraocular pressure in glaucomatous eyes. Br. J. Ophthalmol. 60(1976)111-114.
1. M.D., Francis I. Proctor Foundation, University of California, San Francisco, California 94143-0412.
2. M.D., Francis I. Proctor Foundation, University of California, San Francisco, California 94143-0412.
3. D.P.H., Francis I. Proctor Foundation, University of California, San Francisco, California 94143-0412.
4. M.D., Francis I. Proctor Foundation, University of California, San Francisco, California 94143-0412.
5. San Francisco Regional Hansen's Disease Clinic, San Francisco, California.
Reprint requests to Dr. H. Bruce Ostler.
Received for publication on 16 June 1989.
Accepted for publication in revised form on 6 October 1989.